4921
Effect of menstrual cycle on background parenchymal enhancement and the detectability of breast cancer by contrast-enhanced breast MRI: a multicenter study of an Asian populationTakeshi Kamitani1, Hidetake Yabuuchi2, Mitsuhiro Tozaki3, Yoshihide Kanemaki4, Satoshi Kawanami5, Koji Sagiyama1, Yuzo Yamasaki1, Seitaro Shin1, and Hiroshi Honda1
1Department of Clinical Radiology, Kyushu University, Fukuoka, Japan, 2Department of Health Sciences, Kyushu University, Fukuoka, Japan, 3Department of Radiology, Sagara Hospital Affiliated Breast Center, Kagoshima, Japan, 4Breast and Imaging Center, St. Marianna University School of Medicine, Kawasaki, Japan, 5Department of Molecular Imaging & Diagnosis, Kyushu Universtiy, Fukuoka, Japan
Synopsis
Background parenchymal enhancement (BPE) on breast contrast MRI is known to be associated with the menstrual cycle. We conducted a multicenter study to evaluate the effect of the menstrual cycle on BPE and cancer detectability by contrast-enhanced breast MRI in an Asian population. The Degrees of BPE and cancer detectability were assessed quantitatively and qualitatively. BPE were strongest in the proliferative phase. The detectability of breast cancer was better at the menstrual and proliferative phases than at secretary phase. Not only the proliferative phase but also the menstrual phase is suitable for breast MRI examinations of premenopausal Asian women.
Purpose
Background parenchymal enhancement (BPE) on breast contrast magnetic resonance imaging (MRI) is known to be associated with the menstrual cycle 1-3. Prominent BPE could disturb lesion detection 4,5. Enhancement of normal breast tissue could result in false-positive findings or even mask a small abnormal enhancing cancer, resulting in a false-negative report 1. It is thus important to perform breast MRI at the optimal phases of the menstrual cycle. The European Society of Breast Imaging guidelines state that the optimal time to perform a breast MRI in premenopausal women is between the 5th and 12th day after the menstrual cycle’s start 2, but this is based on studies conducted mostly in Western populations 6-8. To our knowledge, there are only two single-center studies providing corresponding Asian data 9,10. Here we conducted a multicenter study to evaluate the effect of the menstrual cycle on BPE and cancer detectability by contrast-enhanced breast MRI in an Asian population.Methods
We enrolled 962 women who underwent breast MRI in November 2013–March 2015 from 23 centers. The menstrual status was obtained by interview; 266 premenopausal patients (ages 23–54 yrs, mean 42.4 yrs) with regular menstrual cycles were included, and 176 of them were diagnosed by pathology as having breast cancer. Thirty-five patients were examined in the menstrual phase (days 1–4), 105 in the proliferative phase (days 5–14), and 126 in the secretory phase (days 15–30). For a quantitative assessment, the signal intensities (SIs) of breast tissue on the unaffected side on a pre-contrast image (SI1) and an early-phase image (SI2), the SIs of breast tissue on the affected side on a pre-contrast image (SI3) and an early-phase image (SI4), and the SIs of breast cancer on a pre-contrast image (SI5) and an early-phase image (SI6) were measured. We then calculated, for each patient, the BPE ratio, i.e., (SI2 – SI1)/SI1 and the cancer / background enhancement ratio (C/B ratio), i.e., (SI6 – SI5) / (SI4 – SI3). We compared the results of the quantitative assessment with the menstrual cycle using a Kruskal-Wallis analysis. For the qualitative assessment, the BPE was classified as “minimal ” “mild ” “moderate ” or “marked,” and the cancer detectability was classified as “excellent ” “good ” or “poor ” independently by two radiologists. We compared the proportion of minimal-or-mild BPE and that of excellent-or-good cancer detectability with the menstrual cycle using the chi-squared test.Results
The averages of the BPE ratio were 0.25, 0.24, and 0.31 at the menstrual, proliferative, and secretory phases, with no significant differences among these values (p=0.21). The averages of the C/B ratio were 20.1, 15.7, and 9.1 at the menstrual, proliferative, and secretory phases (P<0.001). BPE was determined as moderate or marked in 0% and 5.4% at the menstrual phase, 10.3% and 11.0% at the proliferative phase, and 17.5% and 21.7% at the secretory phase by the two observers, respectively (p=0.01, p=0.01). The detectability of breast cancer was classified as poor in 0% and 0%, 1.4% and 13.0%, and 8.0% and 22.1% at the menstrual, proliferative, and secretory phases by the two observers, respectively (p=0.07, p=0.02).Discussion
Breast tissue is influenced by cyclic hormonal changes, especially estrogen, according to the menstrual cycle 5,10,11. BPE is therefore influenced by the menstrual cycle. A previous study reported a significant correlation between BPE and mammographic density 9. Another study reported that the influence of the menstrual cycle on the BPE is different according to the breast composition, and that the optimal time for breast MRI could be different for dense and fatty breasts; those authors described that fatty breasts showed the highest BPE in the 4th week and lowest BPE in the 2nd week of the menstrual cycle, whereas dense breasts showed the highest BPE in the 3rd week and the lowest BPE in the 4th week 5. Breast composition varies among racial groups. In general, the breasts of Asian women are relatively small in size and are commonly characterized as dense or heterogeneously dense compared to breasts of Western women 12. Our present findings demonstrated that not only the proliferative phase but also the menstrual phase is suitable for determining the optimal timing for breast MRI examinations. The difference in breast composition between Asian women and Western women might be one of the reasons underlying the present acceptable result even in the menstrual phase (1st week).Conclusion
The menstrual cycle affects BPE and the detectability of breast cancer. Not only the proliferative phase but also the menstrual phase is suitable for breast MRI examinations of premenopausal Asian women.Acknowledgements
This study was supported by a grant from Bayer Healthcare.References
1. Ellis RL. Optimal timing of breast MRI examinations for premenopausal women who do not have a normal menstrual cycle. Am J Roentgenol AJR2009;193(6):1738-1740. 2. Mann RM, Kuhl CK, Kinkel K, et al. Breast MRI: guidelines from the European Society of Breast Imaging. Eur Radiol 2008;18(7):1307-1318. 3. Scaranelo AM, Carrillo MC, Fleming R, et al. Pilot study of quantitative analysis of background enhancement on breast MR images: association with menstrual cycle and mammographic breast density. Radiology 2013;267(3):692-700. 4. Shin S, Ko ES, Kim RB, et al. Effect of menstrual cycle and menopausal status on apparent diffusion coefficient values and detectability of invasive ductal carcinoma on diffusion-weighted MRI. Breast Cancer Res Treat 2015; 149(3):751-759. 5. Kang SS, Ko EY, Han BK, et al. Background parenchymal enhancement on breast MRI: influence of menstrual cycle and breast composition. JMRI 2014;39(3):526-534. 6. Delille JP, Slanetz PJ, Yeh ED, et al. Physiologic changes in breast magnetic resonance imaging during the menstrual cycle: perfusion imaging, signal enhancement, and influence of the T1 relaxation time of breast tissue. Breast J 2005;11(4):236–241. 7. Kuhl CK, Bieling HB, Gieseke J, et al. Healthy premenopausal breast parenchyma in dynamic contrast-enhanced MR imaging of the breast: normal contrast medium enhancement and cyclical-phase dependency. Radiology 1997;203(1):137–144. 8. Muller-Schimpfle M, Ohmenhauser K, Stoll P, et al. Menstrual cycle and age: influence on parenchymal contrast medium enhancement in MR imaging of the breast. Radiology 1997;203(1):145–149. 9. Uematsu T, Kasami M, Watanabe J. Should breast MRI be performed with adjustment for the phase in patients’ menstrual cycle? Correlation between mammographic density, age, and background enhancement on breast MRI without adjusting for the phase in patients’ menstrual cycle. Eur J Radiol 2012;81(7):1539-1542. 10. Kajihara M, Goto M, Hirayama Y, et al. Effect of the menstrual cycle on background parenchymal enhancement in breast MR imaging. Magn Reson Med Sci 2013;12(1):39-45. 11. Amarosa AR, McKellop J, Leite APK, et al. Evaluation of the kinetic properties of background parenchymal enhancement throughout the phases of the menstrual cycle. Radiology 2013;268(2):356-365. 12. Takamoto Y, Tsunoda H, Kikuchi M, et al. Role of breast tomosynthesis in diagnosis of breast cancer for Japanese women. Asian Pacific J Cancer Prev 2013;14(5):3037-3040.Figures
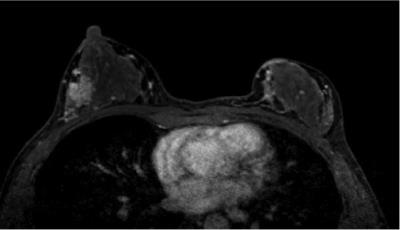
A
41-year-old-woman with invasive ductal carcinoma, in the menstrual phase. The BPE
ratio was 6%, and the qualitative BPE was classified as mild. The C/B ratio was
1349%, and the qualitative cancer detectability was classified as excellent.
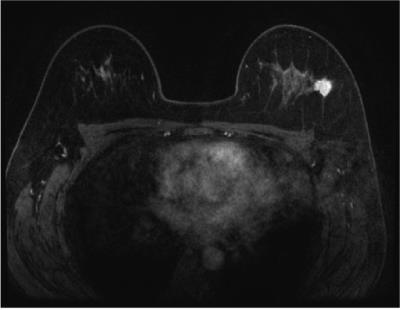
A
47-year-old-woman with invasive ductal carcinoma, in the proliferative phase. The
BPE ratio was 24%, and the qualitative BPE was classified as minimal. The C/B
ratio was 1540%, and the qualitative cancer detectability was classified as excellent.
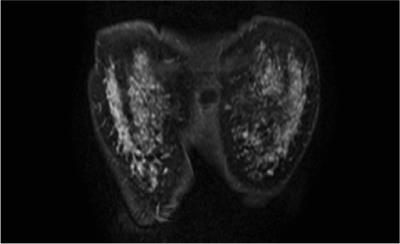
A
48-year-old-woman with non-invasive ductal carcinoma, in the secretory phase. The
BPE ratio was 156% and the qualitative BPE was classified as marked. The C/B
ratio was 119%, and the qualitative cancer detectability was classified as poor.