4913
Development and comparison of 19F and hyperpolarized 129Xe lung MRI for preclinical application in miceAlexandre A Khrapitchev1, Rohan S Virgincar1,2, James R Larkin1, Niloufar Zarghami1, Sheena Wallington1, Ana L Gomes1, Stuart Gilchrist1, Paul Kinchesh1, Sean Smart1, and Nicola R Sibson1
1CR-UK/MRC Oxford Institute for Radiation Oncology, Department of Oncology, University of Oxford, Oxford, United Kingdom, 2Biomedical Engineering, Duke University, Durham, NC, United States
Synopsis
The 19F approach is easier and cheaper to implement than the hyperpolarized gas setup and is immediately applicable to a range of pre-clinical work. However, 129Xe MRI provides a unique dynamic tool that may have important applications warranting the additional effort and cost involved. We have compared lung 19F MRI with hyperpolarized 129Xe MRI, in both cases using an Ultra Short Echo (UTE) sequence on a 7.0T preclinical spectrometer.
Purpose
Imaging plays a major role in assessment of pulmonary diseases, and a broad spectrum of imaging modalities are available. Of these modalities, the main workhorse is still computed tomography (CT), which allows fast and high-resolution assessment of the lung parenchyma and surrounding structures. However, the soft tissue contrast and gain of direct functional information is limited. Lung MRI is difficult owing to the low proton density of the tissue and, consequently, low intensity on conventional 1H MRI. At the same time, large differences in the magnetic susceptibility between lung tissue and air, respectively, reduces the signal strength even further. To overcome these limitations, we have investigated the possibility of imaging mouse lungs by use of fluorinated gases1 such as CF4 and have compared this novel technique with the gold standard hyperpolarized 129Xe MRI2. Biocompatibility, high gyromagnetic ratio of 19F, and extremely short relaxation times (T1 and T2 in order of a few milliseconds) are the key benefits of fluorinated gases. In addition, the low cost of the gases, and no requirement for expensive polarization equipment, make perfluorocarbons ideal for lung MRI. Finally, the absence of fluorine in the body results in the lungs appearing bright with no background signal.Methods
19F and hyperpolarized 129Xe MR images were acquired using an Ultra Short Echo (UTE) sequence on a 7.0T preclinical spectrometer (Agilent Inc, USA). 129Xe images were acquired using a home-built Helmholtz coil, and 19F images with a quadrature coil (PulseTeq, UK). For both nuclei, 3D radial trajectories were used; number of points along each trajectory np=128, and number of trajectories ntraj=8192. The acquired points were gridded onto a 1283 matrix. Although the imaging sequence in both cases was identical, the imaging strategies were different. Hyperpolarized 129Xe MRI used isotopically enriched gas (80% 129Xe) polarized to 20% (Polarian Inc, USA). During each breath-hold, 8 radial trajectories were acquired, with excitation angle a=20°, TR=20ms and TE=10μs, making the total experimental time Texp=16min. For 19F MRI, a very short TR=2ms was used, and 80 trajectories were acquired during each breath-hold, with a typical excitation angle a=60°. The thermally-polarized gases have inherently low signal and, therefore, require significant signal averaging. We limited our total experimental time to Texp=2h, which corresponds to NT=100. Proton images were also acquired using the UTE sequence and the same breath-hold technique, with the following imaging parameters: TR=10ms, TE=10μs, a=20°, NT=1, Texp=16min.Results and Discussions
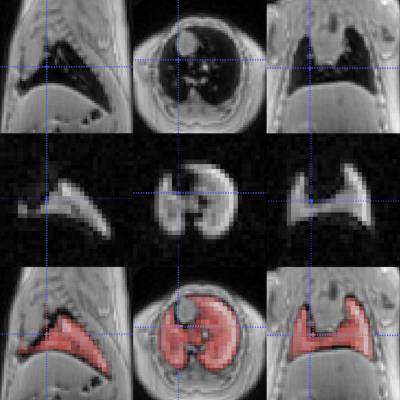
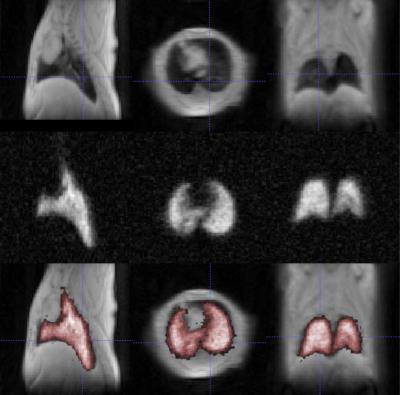
In this pilot study ex vivo naïve mice were used, in which the lungs were ventilated using a custom-built MR- and HP-gas compatible small animal ventilator (Duke University, USA) and data were acquired during breath-hold within an in vivo compatible breathing cycle (50ms – controlled inhalation, 250ms – breath-hold, 450ms – passive exhalation). Good quality images of the lungs were acquired using both 19F (Figure 1) and hyperpolarized 129Xe (Figure 2) MRI techniques, and the typical Signal-To-Noise (SNR) in both cases was broadly similar. Interestingly, the two approaches provide complimentary information; 129Xe MRI can provide dynamic/functional information on the lungs, whilst influx of fresh 19F does not influence the MR Image so much and, therefore, more static/physiological information is obtained. This information, combined with proton MR images, offers a unique lung diagnostic tool and the next step will be to move to in vivo studies, which would require overcoming additional challenges (e.g. replacement of non-recovery tracheotomy with recovery intubation for longitudinal studies; maintenance of the injectable anesthetic depth, by refining initial and supplementary dosage; fine synchronization of various experimental stages, etc.)Conclusion
The ability to monitor lung function is undoubtedly important. 19F based perfluorocarbon and hyperpolarized 129Xe MRI provide bright and detailed lung images, which enable visualization of lung structure and function to a degree that is not possible with proton MRI. The 19F approach is easier and cheaper to implement than the hyperpolarized gas setup and is immediately applicable to a range of pre-clinical work. However, 129Xe MRI provides a unique dynamic tool that may have important applications warranting the additional effort and cost involved.Acknowledgements
This work was supported by Cancer Research UK (grant number C5255/A12678) and the CRUK & EPSRC Cancer Imaging Centre in Oxford (grant number C5255/A16466). The authors also thank Stavros Melemenidis (Stanford University, USA), Peter Thelwall (Newcastle University, UK) and Konstantinos Papoutsis (MR Solutions, UK) for their time and effort on the initial stages of this long lasting journey.References
1. Kuethe et al. “Imaging lungs using inert fluorinated gases”. Magn Reson Med. 1998 Jan; 39(1): 85-8.
2. Albert et al. “Biological magnetic resonance imaging using laser-polarized 129Xe”. Nature. 1994 Jul; 370(6486): 199-201.