4909
Spatial Tagging to Assess Regional Ventilation of Lung Parenchyma with Endogenous ContrastEamon Doyle1,2, Roberta Kato3, Jonathan M Chia4, and John C Wood1,2
1Biomedical Engineering, University of Southern California, Los Angeles, CA, United States, 2Cardiology, Children's Hospital of Los Angeles, Los Angeles, CA, United States, 3Pediatrics, Children's Hospital of Los Angeles, Los Angeles, CA, United States, 4Philips Healthcare, Gainesville, FL, United States
Synopsis
Tagging techniques such as SPAMM and CSPAMM have been useful for assessment of dynamic cardiac tissue deformation. Recent advances in ultra-short echo time (UTE) imaging have enable imaging of lung parenchyma. In this work, we evaluate the possibility of using spatial tags in conjunction with UTE imaging to assess regional ventilation and tissue stiffness with non-enhanced, endogenous contrast.
Introduction
Regional ventilation in the lung is a sought-after diagnostic measurement with long-standing challenges.[1] Magnetic resonance imaging has traditionally failed in the lung due to low proton density, significant magnetic heterogeneity, and susceptibility causing complete loss of signal in the parenchyma.[2] However, MRI is attractive compared to other modalities due to the many contrast mechanisms, good spatial and temporal resolution, and lack of ionizing radiation. Pediatric patients particularly benefit from MRI because they are more susceptible to complications from exposure to radiation than adults.[2] Successful lung imaging with hyperpolarized gas can provide good spatial resolution but is restricted to research settings due to the high cost and infrastructure requirements.[3] Recently, ultra-short echo time (UTE) imaging has been successful at regaining contrast in the lungs, allowing for anatomical assessment.[4] In this work, we investigate the possibility of using UTE imaging pulse sequences combined with cardiac tagging techniques, SPAMM[5] and CSPAMM, and respiratory Navigation for noninvasive estimation of regional ventilation and tissue stiffness noninvasively with endogenous contrast.Methods
Images were acquired as part of an IRB-approved study (CHLA CCI-12-00095) in four healthy volunteers. A Philips Ingenia CX 3T magnet (Release 5.1.9 SP1) with a dStream Torso coil was used to acquire images with a 3D UTE radial stack-of-stars readout with the following imaging parameters: TE=150 μs, flip angle=8 degrees, spoke density=100%, pixel=2x2mm, slice=8mm, FOV=350x350mm, SENSE factor = 2 (slice), NSA = 1 (2 for CSPAMM). Number of slices was selected on a per-subject basis to achieve full coverage. Custom pulse sequences were developed with the Philips pulse programming environment; SPAMM or CSPAMM spatial saturation pulses were prepended to the UTE imaging scheme, creating a saturation grid or saturation lines in the imaging stack. The acquisition readout was triggered at end-expiration with a Navigator beam on the diaphragm to reduce respiratory ghosting.Results
Successful tagging was achieved in four subjects. Representative images in each of the planes demonstrated that tagging in the lung parenchyma provides sufficient contrast to enable lung strain estimation (figure 1). SPAMM tags were found to be more robust than CSPAMM tags (figure 2). The off-resonance effects caused some tag blurring in SPAMM images but intersections of orthogonal tag lines remained visible. Manual comparison of the grid spacing in the posterior lungs with spacing in arm muscle and subcutaneous fat showed variations of less than 2%. Blurring and tag deformation in the CSPAMM tags were more severe. Increased tag width led to more noticeable warping of the tag in the parenchyma.Discussion
To our knowledge, this is the first successful attempt to perform SPAMM and CSPAMM tagging in the lung using endogenous contrast. The combination of SPAMM and CSPAMM tags with the UTE readout appear to enable regional strain analysis of the lung parenchyma, paving the way for rapid, non-invasive tissue stiffness mapping of the lung. Tissue strain has the potential to demonstrate ventilation and reveal tissue compliance abnormalities that may be indicative of disease. While many attempts to image lung parenchyma have been made with hyperpolarized helium and xenon or 100% oxygen, reducing dependence on exogenous contrast will lead to more rapid and widespread application in clinical settings. Although only one tag delay was demonstrated in this work, the successful capture of cardiac motion over the imaging window supports the likely success of our approach. Limitations related to trigger timing prevented the gathering of multiple delay times between the trigger and the imaging, which would demonstrate dynamic compliance throughout the respiratory cycle. However, we are actively developing both external and internal triggering and gating mechanisms to improve the acquisition timing and creating an offline reconstruction pipeline to sample the entire respiratory cycle and better utilize the resulting data.Conclusion
Combining SPAMM and CSPAMM tags with UTE imaging and respiratory demonstrates successful spatial tagging in lung parenchyma without exogenous contrast enhancement; these techniques show promise for clinical evaluation of regional ventilation and tissue stiffness. The addition of more robust gating, triggering, and reconstruction techniques will significantly extend the usefulness of these techniques in the lung.Acknowledgements
This work is supported by the National Institute of Health NIDDK National Institute of Diabetes and Digestive and Kidney Diseases by grant 5R01DK097115-03 and the Webb Foundation for Cystic Fibrosis. Clinical science support is provided by Philips Healthcare. Research space and computational resources are generously provided by Dr. Krishna Nayak and the Magnetic Resonance Engineering Lab.References
1. Wagner PD, Dantzker DR, Dueck R, Clausen JL, West JB. Ventilation-perfusion inequality in chronic obstructive pulmonary disease. Journal of Clinical Investigation. 1977;59(2):203–216. 2. Ciet P, Tiddens HAWM, Wielopolski PA, Wild JM, Lee EY, Morana G, Lequin MH. Magnetic resonance imaging in children: common problems and possible solutions for lung and airways imaging. Pediatric Radiology. 2015 Sep 5 [accessed 2015 Oct 9]. http://link.springer.com/10.1007/s00247-015-3420-y 3. Liszewski MC, Hersman FW, Altes TA, Ohno Y, Ciet P, Warfield SK, Lee EY. Magnetic Resonance Imaging of Pediatric Lung Parenchyma, Airways, Vasculature, Ventilation, and Perfusion. Radiologic Clinics of North America. 2013;51(4):555–582. 4. Johnson KM, Fain SB, Schiebler ML, Nagle S. Optimized 3D Ultrashort Echo Time Pulmonary MRI. Magnetic resonance in medicine?: official journal of the Society of Magnetic Resonance in Medicine / Society of Magnetic Resonance in Medicine. 2013;70(5):1241–1250. 5. Fischer SE, McKinnon GC, Maier SE, Boesiger P. Improved myocardial tagging contrast. Magnetic Resonance in Medicine. 1993;30(2):191–200.Figures

A representative set of acquisitions with
SPAMM grid tag. Full size (left) and cropped (right) images demonstrate
successful SPAMM tags in the (top to bottom) transverse, saggital, and coronal
planes. Tag line deflection caused by cardiac motion and blood flow
indicate that the temporal resolution of this technique will be sufficient for
observing deflection in lung parenchyma.
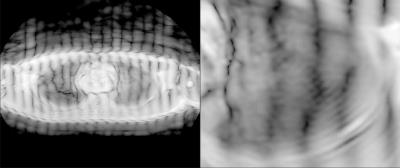
A demonstration of successful CSPAMM tag
lines in the transverse plane. The achieved CSPAMM tag was less robust to
the high susceptibility environment, producing more blurring and warping as
well as reduced edge definition. Nonetheless, we believe that tuning the
sequence will lead to improvements in tag quality. Image log-transformed to improve grayscale
contrast.