4908
High Spatiotemporal Resolution 4D-MRI for Evaluation of Spatially Resolved Pulmonary Function1Center of Advanced Imaging Innovation and Research (CAI2R), Department of Radiology, New York University School of Medicine, New York, NY, United States, 2Bernard and Irene Schwartz Center for Biomedical Imaging, Department of Radiology, New York University School of Medicine, New York, NY, United States, 3Department of Radiology, University Hospital (CHUV) and University of Lausanne (UNIL), Lausanne, Switzerland.
Synopsis
Spirometry provides global measures of lung function, whereas CT provides morphologic information but limited functional information. Current tests are limited in providing spatially resolved function of each lung separately. To address this need, we have developed a 4D ultra-short echo time MRI method where respiratory motion information is extracted directly from the acquired k-space data during normal and deep breathing maneuvers, and motion-resolved reconstruction is performed to extract spatially resolved functional information of each lung. Such a method will improve the ability to diagnose and manage Chronic Obstructive Pulmonary Disease, which is a major cause of death worldwide.
INTRODUCTION
Chronic Obstructive Pulmonary Disease (COPD) is a significant cause of morbidity and mortality worldwide. COPD is a heterogeneous disease with respect to spatial distribution of the disease and clinical presentation. Although pulmonary function tests (PFTs), measured by spirometer are required to make a diagnosis of irreversible airway obstruction, these tests are inadequate in assessing disease heterogeneity and provide no spatial information1-3. CT provides excellent spatial morphologic information, but limited functional information. Conventional MRI permits continuous scanning, due to lack of radiation exposure, enabling data acquisition during the entire respiratory cycle. However, it suffers from low SNR and low acquisition speed for volumetric 4-dimensional (4D) imaging.
To overcome these limitations, we have developed a UTE (ultra-short echo time) method that continuously acquires data during breathing, and reconstructs a series of images with whole-lung volumetric coverage over different respiratory phases. Respiratory motion information is extracted directly from the acquired k-space data, and motion-resolved reconstruction is performed using a compressed sensing approach. In this study, we acquired volumetric 4D images of the lung with high spatiotemporal resolution during normal and deep breathing maneuvers. From these images, spatially resolved functional information of each lung was extracted which is a not easily possible using conventional method.
METHODS
Subjects: 7 volunteers and 3 patients were recruited for this prospective IRB-approved study. 4 volunteers were imaged twice in two separate visits to assess scan-rescan reproducibility in MR measures of pulmonary function (PF-MR).
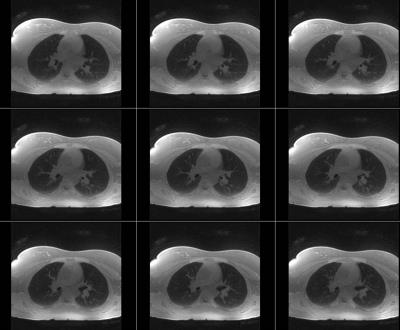
Acquisition: Center-out half-projection radial k-space data were continuously acquired using a UTE sequence described previously4. The k-space measurements were segmented into multiple interleaves rotating at a golden angle for respiratory motion detection as previously described5. Imaging parameters included: TR/TE=3.3/0.05ms, matrix size=1283, voxel size≈ (2mm)3. Two consecutive acquisitions were performed. First the subjects were asked to breathe normally. A total of 61260 spokes and 1021 interleaves were acquired in 4 minutes. For the second acquisition, subjects were instructed to breathe normally for one minute, followed by a deep breathing maneuver for another one minute, during which they were instructed to inhale as-deeply-as possible then exhale as-rapidly-as possible. These one-minute-normal and one-minute-deep breathing cycles were repeated 4 times. A total of 122,520 spokes and 2042 interleaves were acquired in 8 minutes. All data from the first scan were used for reconstructing the normal breathing image series, and the data acquired during deep breathing in the second scan (net scan time=4 minutes) were used for reconstructing a deep-breathing image series. k-space data were sorted into 30 respiratory phases from expiration to inspiration and then back to expiration, covering an entire respiratory cycle using the extracted respiratory motion signal Followed by XD-GRASP reconstruction of the sorted highly-undersampled 4D dataset (kx-ky-kz-respiratory)5 (Figure 1).
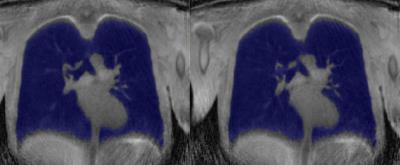
Post-processing: Right and left lung were semi-automatically segmented using the in-house software (wp.nyu.edu/FireVoxel) by two readers (in 7 subjects). The volume of each lung in each respiratory phase for normal breathing and deep breathing was computed (Figure 2). Right, left, and global PF-MR parameters were obtained and the global PF-MR FEV1/FVC was compared to FEV1/FVC measured with spirometry.
RESULTS
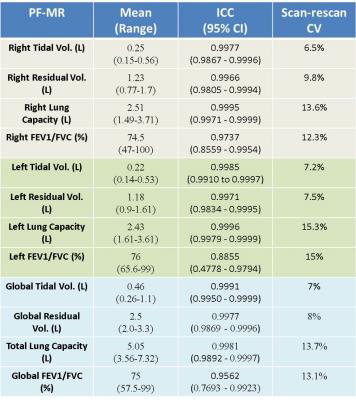
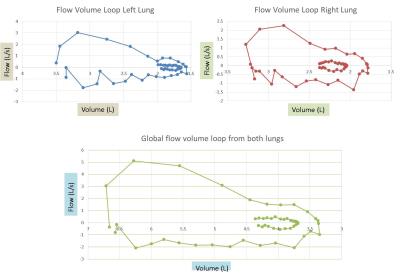
The PF-MR parameters: Tidal volume (TV), residual volume (RV), lung capacity (LC), and FEV1/FVC were obtained in the 10 subjects for the right and the left lung individually, and they were also combined to obtain global measures (Figure 3). The flow-volume loop for each lung individually and for both lungs together is shown in Figure 4 for one of the subjects.
Inter-reader agreement (Figure3): PF-MR measures were independently obtained by two readers. There was excellent inter-reader agreement in right, left, and global measures of tidal volume, residual volume, lung capacity, and FEV1/FVC, with an intra-class correlation coefficient ranging from 0.8855 to 0.9995.
Scan-rescan variability (Figure 3): 4 subjects underwent a second MR examination. The scan-rescan variability in right, left, and global measures of PF-MR was computed with coefficient of variance ranging from 6.5% to 15.2% for various PF-MR parameters.
PF-MR to Spirometry comparison: The spirometry measure of FEV1/FVC was available in six subjects. The correlation between the MR and spirometry measure of FEV1/FVC was 0.724.
DISCUSSION & CONCLUSION
We have developed a method that simultaneously provides structural and functional information of each lung. There was excellent inter-reader agreement in PF-MR measures with low scan-rescan variance. Furthermore, there was moderate to strong correlation between MR and spirometry FEV1/FVC.
Our initial study suggests that the proposed 4D-MRI approach can be extended to provide spatially resolved functional information at a lobar or segmental level which may improve diagnosis and management of patients with various lung diseases with heterogeneous distribution such as COPD.
Acknowledgements
Part of this research was funded by NIH 5R01EB018308 and P41EB017183References
1. Han MK et al. Chronic obstructive pulmonary disease phenotypes: the future of COPD. Am J Respir Crit Care Med. 2010 Sep 1;182(5):598-604.
2. Ford ES et al. Total and state-specific medical and absenteeism costs of COPD among adults aged ≥ 18 years in the United States for 2010 and projections through 2020. Chest. 2015 Jan;147(1):31-45.
3. Miravitlles M et al. Clinical phenotypes of COPD: identification, definition and implications for guidelines. Arch Bronconeumol. 2012 Mar;48(3):86-98.
4. Delacoste J et al. A double echo ultra short echo time acquisition for respiratory motion suppressed high resolution imaging of the lung. ISMRM 2015: p1455.
5. Feng L et al Four-Dimensional Respiratory Motion-Resolved Sparse Lung MRI. ISMRM 2016: p 2918.
Figures