4904
Accelerated Stack-of-Spirals Breath-hold UTE Lung Imaging1Radiology & Medical Imaging, University of Virginia, Charlottesville, VA, United States, 2Biomedical Engineering, University of Virginia, Charlottesville, VA, United States, 3Application Development, Siemens Healthcare, Erlangen, Germany
Synopsis
Stack-of-spirals and cones trajectories have been proposed to permit breath-hold ultrashort-echo-time (UTE) acquisitions of the human lung, although further acceleration would be valuable to permit improvements such as shorter breath-holds for respiratory comprised patients or higher spatial resolution. In this work, an accelerated UTE 3D stack-of-spirals pulse sequence was implemented using undersampled, dual-density spiral waveforms for acquisition and a SPIRiT-based algorithm for image reconstruction. Preliminary testing in healthy volunteers using 2-fold acceleration provided image quality comparable to that achieved with the original, unaccelerated pulse sequence.
Introduction & Purpose
Substantial progress has been made recently in using ultrashort-echo-time (UTE) techniques for imaging the lung, with its intrinsically short T2* relaxation time. While most work has focused on 3D radial-based methods1,2, these acquisitions are comparatively slow because radial k-space trajectories are relatively inefficient. Stack-of-spirals and cones trajectories have been proposed to permit breath-hold UTE acquisitions3,4, although further acceleration would be valuable to permit improvements such as shorter breath-holds for respiratory comprised patients or higher spatial resolution. The purpose of this work was to develop and perform preliminary testing of an optimized 3D stack-of-spirals acquisition4 that supports in-plane acceleration.Methods
The prototype UTE 3D stack-of-spirals acquisition was based on a commercial version of 3D spoiled gradient-echo imaging (VIBE, Siemens Healthcare, Erlangen, Germany), modified as described in ref. 4 to support a stack-of-spirals acquisition and a very short TE, which was achieved by using non-spatially-selective RF excitation and by minimizing the duration of each through-plane phase-encoding gradient waveform while simultaneously minimizing the TE5. This permitted a TE of 50 µs for the central k-space plane. In-plane acceleration was implemented by acquiring a reduced number of spiral interleaves for each k-space plane (e.g., every second or third interleave), and reconstructing the resulting data using a SPIRiT6-based algorithm implemented on the MR scanner. For image reconstruction, 3D data were first Fourier transformed along the third dimension, and a SPIRiT-based reconstruction was then performed on each plane of undersampled spiral data. Dual-density spiral waveforms were used to provide fully sampled data for the central portion of each k-space plane, and undersampled data for the remainder.
The accelerated UTE 3D stack-of-spirals acquisition was tested on 1.5T (MAGNETOM Aera, Siemens Healthcare, Erlangen, Germany) and 3T (MAGNETOM Prisma, Siemens) MR scanners in phantoms and in healthy volunteers, after obtaining informed consent. Compared to the original UTE 3D stack-of-spirals acquisition, the acceleration was used in subjects to perform breath-hold whole-lung acquisitions with a reduced acquisition time, to achieve increased spatial resolution during approximately the same acquisition time, and to permit double-echo imaging with reduced acquisition time.
Results
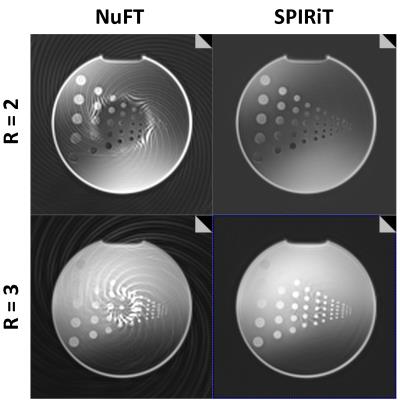
The performance of the acquisition and reconstruction algorithm for 2- and 3-fold acceleration is illustrated with phantom images in Fig. 1. The left column presents images from direct reconstruction of the undersampled data, showing obvious undersampling artifacts. The right column presents images from the SPIRiT-based algorithm; 2-fold acceleration shows essentially no artifacts, and 3-fold acceleration shows only very minor remaining artifacts.
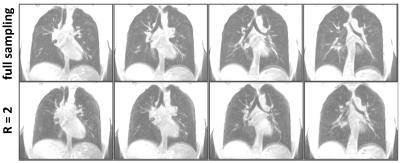
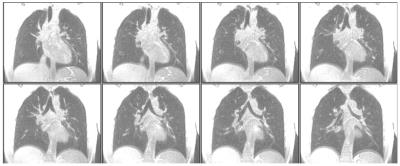
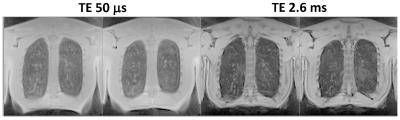
The original UTE 3D stack-of-spirals acquisition could provide spatial resolution of approximately 2 x 2 x 5 mm3 for the adult chest from a 16-s breath-hold acquisition. Figure 2 compares results from the original technique to those from 2-fold acceleration, providing the same spatial resolution from an 8-s breath-hold acquisition. The image quality appears very similar for the original and accelerated acquisitions. Figure 3 shows results from the same subject wherein the acceleration was used to increase spatial resolution to 2 x 2 x 2.5 mm3 for an acquisition time of 17 s. Finally, Fig. 4 illustrates acquisition of two echo times (50 µs and 2.6 ms) during a 9-s breath hold, and shows a decrease in signal intensity of the lung tissue with the relatively small 2.6-ms increase in echo time, as expected.
Conclusions & Future Work
An accelerated UTE 3D stack-of-spirals pulse sequence was implemented using undersampled, dual-density spiral waveforms for acquisition and a SPIRiT-based algorithm for image reconstruction. Preliminary testing in healthy volunteers using 2-fold acceleration provided image quality comparable to that achieved with the original, unaccelerated pulse sequence. Future work will focus on testing in subjects with lung disease, and on implementing acceleration in the third (Cartesian) direction to permit higher acceleration factors. Our approach should also be compared to alternatives such as accelerated 3D cones3.Acknowledgements
No acknowledgement found.References
1. Johnson KM et al. Magn Reson Med 2013; 70:1241-1250.
2. Miller GW et al. NMR Biomed 2014; 27:1542-1556.
3. Zeimpekis K et al. Proc Intl Soc Mag Reson Med 2016; 24:1608.
4. Mugler JP et al. Proc Intl Soc Mag Reson Med 2015; 23:1476.
5. Qian Y, Boada FE. Magn Reson Med 2008; 60:135-145.
6. Lustig M, Pauly JM. Magn Reson Med 2010; 64:457-471.
Figures