4903
Hyperpolarised 3He Diffusion-Weighted MRI in Mild Cystic Fibrosis Children and Age-matched Healthy Controls1Academic Unit of Radiology, University of Sheffield, Sheffield, United Kingdom, 2Sheffield Children's Hospital, Sheffield, United Kingdom, 3Manchester Adult CF Centre, Manchester, United Kingdom
Synopsis
Hyperpolarised gas MRI is sensitive to lung ventilation heterogeneity in early cystic fibrosis (CF) disease. However, lung microstructural changes that might accompany early lung disease in CF is less well explored. DW-MRI measurements were compared in mild CF children and age-matched healthy controls, and reassessed after a 2-year interval in the CF group. No significant difference in DW-MRI metrics (in contrast to changes in lung ventilation, VD%) was observed between healthy controls and CF children, and between baseline and 2-year follow-up visits. These results suggest that no acinar microstructural changes occur in early stage CF despite increases in ventilation heterogeneity.
Purpose
Hyperpolarised (HP) 3He and 129Xe gas imaging has been shown to be sensitive to lung ventilation heterogeneity in early cystic fibrosis (CF) disease. 1,2 FEV1 (forced expiratory volume in 1 second) is the current clinical standard for assessing lung disease severity in CF, but it is insensitive to early changes. 1 However, to date, little is known about changes in lung microstructure that accompany this disease progress. HP diffusion-weighted (DW) MRI has been shown to be sensitive to changes in lung microstructure at the acinar level. 3 This work aims to compare 3He DW-MRI metrics in mild CF children and age-matched healthy controls, and to reassess microstructural metrics after a 2-year interval in the same group of CF children.Methods
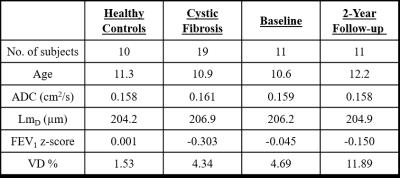
19 CF children with normal spirometry (FEV1 z-score > -1.64) and 10 age-matched healthy controls were assessed with HP gas MRI (3He ventilation and DW imaging) and same-day spirometry. To date, 11 of the CF children have returned for a 2-year follow-up visit. All HP gas MRI was performed on a 1.5T GE HDx scanner using 2D SPGR 3He ventilation and multiple b-value DW sequences described previously. 4,5 3He doses (~25% polarisation) were scaled according to subjects’ height (150-250 mL 3He, and were mixed with N2 in a 1:1 ratio).
Apparent diffusion coefficient (ADC) and estimates of alveolar dimension (LmD) derived from the stretched exponential model 6 were calculated voxel-wise, and a global mean value was obtained for each subject. Quantitative lung microstructural metrics derived from DW-MRI, along with the ventilation defect percentage (VD %) calculated from ventilation imaging 1 and FEV1 z-score were compared between the healthy controls and CF patients, and between baseline and 2 year follow-up visits.
Results and Discussion
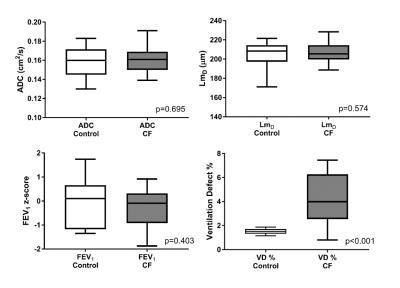
No significant difference in mean ADC, LmD, and FEV1 z-score was observed between healthy controls and CF children (Table 1 and Figure 1). In contrast, VD % was significantly larger (p<0.001) in CF children. The global mean ADC value for both groups was ~0.160 cm2/s; which matches values previously reported in healthy subjects of this age range at 1.5T (0.158 cm2/s). 7 The LmD parameter is similar to the mean linear intercept length obtained from histological lung samples. The mean LmD value for the children in this study was ~205 μm, and this was comparable to the average intercept distance obtained from explanted paediatric lungs of a similar age (~215 μm). 8
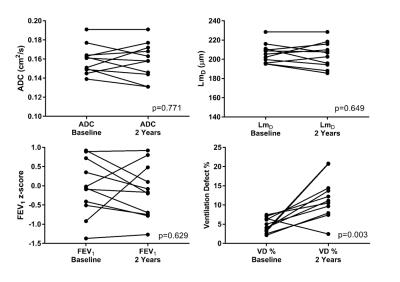
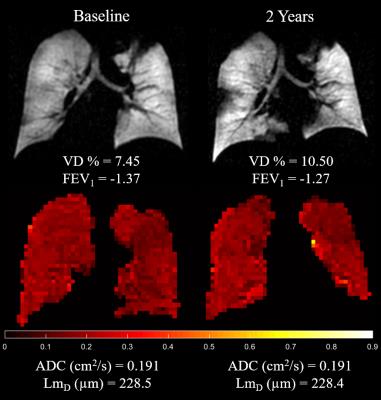
After 2 years, mild CF children did not show a significant change in ADC, LmD, and FEV1 z-score; while a significant increase in VD % was observed (p=0.003) (Table 1 and Figure 2). Figure 3 demonstrates this trend in comparative static ventilation images and ADC maps at baseline and 2-year follow-up in the same patient. These results indicate that despite a significant increase of ventilation heterogeneity (measured by increased VD %) in CF patients at baseline and after 2-years, no accompanying lung microstructural changes at the acinar level were observed. However, it is important to note that HP gas DW-MRI is limited by its ability to assess lung microstructure in ventilated regions only, therefore no diffusion-weighted metrics were measured in the non-ventilated lung regions.
This study found no difference in whole lung ADC at baseline and 2-year follow-up visits, but a statistically significant increase in anterior to posterior (A-P) ADC gradient (p=0.027) was however observed after 2-years. A previous longitudinal study of adult CF subjects scanned at baseline and after 1 week found statistically significant decreases in both whole lung ADC and A-P ADC gradient after 1 week. 9 This discrepancy in ADC results could be explained by the differences in CF population. Adult CF patients compared to mild CF children reflect more advanced lung disease that can be detected with HP gas DW-MRI.
Conclusion
CF is a small airways disease where early ventilation heterogeneity is thought to be caused by mucous plugging. In patients with early CF lung disease, acinar microstructural changes were not seen to accompany early ventilation heterogeneity.Acknowledgements
No acknowledgement found.References
1. Marshall, H., et al. (2014). ERJ 44 (Suppl 58).
2. Thomen, R. P., et al. (2016). J Cyst Fibros. doi: 10.1016/j.jcf.2016.07.008.
3. Saam, B. T., et al. (2000). Magn Reson Med 44(2): 174-179.
4. Horn, F. C., et al. (2014). J Appl Physiol (1985) 116(2): 129-139.
5. Parra-Robles, J., et al. (2012). Magn Reson Med 67(2): 322-325.
6. Parra-Robles, J. et al. (2014). Proc ISMRM:3529.
7. Altes, T. A., et al. (2006). J Magn Reson Imaging 24(6): 1277-1283.
8. Thurlbeck, W. M. (1982). Thorax 37(8): 564-571.
9. Kirby, M. (2013). Am J Respir Crit Care Med 2013; 187:A2072.
Figures