4900
Impact of Helium-Oxygen Inhalation on Aerosol Deposition in Asthmatic Rats using UTE-MRI1Imagerie par Résonance Magnétique Médicale et Multi-Modalités (UMR8081) IR4M, CNRS, Univ. Paris-Sud, Université Paris-Saclay, Orsay, France, 2Centre de Recherches Biologiques, CERB, Baugy, France, 3Centre d'Imagerie du Petit Animal CIPA, PHENOMIN-TAAM UPS44, CNRS, Orléans, France
Synopsis
Asthma is a worldwide chronic respiratory disease. The common treatment by inhaled therapy needs quantitative imaging approaches to understand the impact of carrier gas on aerosol deposition. 3D UTE-MRI combined with aerosolized Gd-DOTA was applied onto spontaneous breathing and mechanically ventilated asthmatic animals. Here, administration and imaging protocols were developed to ventilate and nebulize control and asthmatic rats in order to compare the resulting aerosol distribution with two carrier gas mixtures: air and helium-oxygen.
INTRODUCTION
Asthma is a worldwide chronic respiratory disease. The common treatment by inhaled therapy1 needs quantitative imaging approaches to understand the impact of carrier gas on aerosol deposition. 3D UTE-MRI combined with aerosolized Gd-DOTA2 was applied onto spontaneous breathing3 and mechanically ventilated4 asthmatic animals. Here, administration and imaging protocols were developed to ventilate and nebulize control and asthmatic rats in order to compare the resulting aerosol distribution with two carrier gas mixtures: air and helium-oxygen.METHODS
Animal model: Eleven ovalbumin-sensitized (OVA; 1mg/rat)5 female Wistar rats, (298±13)g, with an increase of airway resistance indicator (PenH)6 verified by plethysmography following OVA-challenge. Before imaging, OVA-sensitized rats were challenged by 20-minute OVA-nebulization to trig asthmatic symptoms, which appeared 2h-post and lasted >2h. Nine control, (299±11)g, were nebulized by 20-minute saline solution.
Administration protocol was carried out with nose-only mask2 (5 control vs. 9 OVA) and with MR-compatible Small Animal Gas Administration System (SAGAS)4 (4 control vs. 2 OVA). Mechanical ventilation was tuned to match individual free-breathing patterns (45-60 breath/min), with controlled breathing cycles, ventilation volumes and inspiration-synchronized nebulization.
Imaging protocol was performed on a clinical 1.5T [Philips Achieva] with a 47mm-diameter receiver coil2. Rats, anesthetized by isoflurane in supine position, breathed spontaneously through the mask during free-breathing, and were tracheotomized with an intratracheal catheter connected to SAGAS. 3D isotropic T1W-UTE radial acquisitions (TR/TE=14/0.4ms, (0.5mm)3 voxel, Tacq=7.5min) were performed once respectively before, during and after nebulization. Gd-DOTA [Dotarem; Guerbet] was nebulized on line [Aeroneb Solo; Aerogen] during ~7min to the rats. Same protocol was repeated successively with air and then helium-oxygen, vice versa.
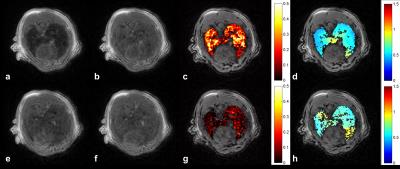
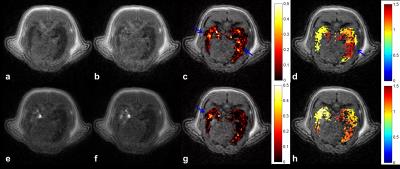
Image analysis: Relative signal enhancement (RSE) at post-contrast within segmented lung region-of-interest2, was converted into concentration maps2,7. Global aerosol distribution was evaluated in the entire lungs using relative dispersion RD (ratio of standard deviation to mean). Regional distribution was assessed by relative concentration deposited in 2 equal volumes (V1=V2) along 4 anatomical directions (Left/Right, Anterior/Posterior, Feet/Head, CEnter/PEriphery). Local heterogeneity was further assessed to investigate local airway collapse8. To do so, a voxel-wise heterogeneity index (HIi,j,k) within the lungs was calculated, by RD of fractional concentration in a cubic kernel of length scale L (10% of the average of left and right lung maximum width). Group analysis was expressed as mean ± standard error of the mean (SEM) and statistical analysis was performed by unpaired two-tail t-tests with a significance level of 0.05.
RESULTS
Under air-free-breathing, lower aerosol concentration was observed in asthmatic rats compared to controls, (0.09±0.01)mM vs. (0.15±0.04)mM. A significantly more heterogeneous distribution was found in asthmatic group, by RD (1.73±0.27) vs. (0.98±0.14) (p<0.05) and average HI (1.15±0.02) vs. (0.72±0.03) (p<0.01). Correlation was obtained between RD and PenH (R= 0.61, p<0.01), as well as HI and PenH (R=0.63, p<0.01) in asthmatic lungs. Under helium-oxygen free-breathing, comparable deposited aerosol (0.09±0.01)mM vs. (0.10±0.03)mM, and corresponding RD (1.96±0.29) vs. (1.95±0.70), were observed. Asthmatic rats had a decreasing HI compared to air-breathing, but slightly higher than control (1.04±0.07) vs. (0.75±0.07) (p<0.05). For regional analysis, a relative homogeneous distribution was observed with air; while A/P heterogeneity in control, and L/R, A/P, CE/PE heterogeneity (>10% difference) in asthmatic rats breathing helium-oxygen were observed.
Through SAGAS with air, asthmatic rats had a lower global RD, (2.4±0.97) vs. (3.30±0.86), but a higher local HI (1.34±0.36) vs. (1.16±0.06). Higher heterogeneity in control with SAGAS4 was revealed here. During breathing helium-oxygen, a comparable RD was obtained between two groups, (2.52±0.17) vs. (2.67±1.67), while a higher local heterogeneity was always present within asthmatic rats, (1.38±0.09) vs. (1.19±0.23). In both carrier gases, asthmatic rat with higher PenH had higher RD and HI. Regional analysis via SAGAS showed L/R heterogeneity in control and A/P, F/H, CE/PE in asthmatic ones with air; F/H heterogeneity in control and L/R, A/P, F/H heterogeneity in asthmatic rats with helium-oxygen.
DISCUSSION AND CONCLUSION
The bronchoconstriction-induced heterogeneous deposition9 in asthmatic lungs and its correlation with PenH were again confirmed in both breathing modalities4, by global and local dispersion. High inter-subject variability was observed in regional aerosol deposition. CE/PE heterogeneity observed in helium-oxygen-breathing asthmatic rats may be related to deeper particle deposition, compared with air10. During free-breathing, aerosol distribution in asthmatic lungs was more homogenous when breathing helium-oxygen than breathing air. Through SAGAS, same trend was observed. However, gas particle interaction could be complicated in the breathing circuit4 and rather small population limited significant finding. Despite inter-subject difference, more homogeneous and deeper deposition was shown with helium-oxygen. The imaging method, with small aerosol dose in clinical MRI, quantitatively maps deposition in global, regional and voxel-wise scales within the lungs.Acknowledgements
This work is part of the OxHelease project (grant ANR-11-TecSan-006-02) and is supported by the Physics and Imaging for Medicine program at Université Paris-Saclay. Imaging was performed on the 1.5 T MRI platform at SHFJ (Orsay, France), partly funded by France Life Imaging (grant ANR-11-INBS-0006).References
1. Capstick TG, Clifton IJ. Inhaler technique and training in people with chronic obstructive pulmonary disease and asthma. Expert review of respiratory medicine 2012;6:91-101; quiz 102-103.
2. Wang H, Sebrie C, Ruaud JP, Guillot G, Bouazizi-Verdier K, Willoquet G, Maitre X, Darrasse L, de Rochefort L. Aerosol deposition in the lungs of spontaneously breathing rats using Gd-DOTA-based contrast agents and ultra-short echo time MRI at 1.5 Tesla. Magn Reson Med 2016;75:594-605.
3. Wang H, Sebrié C, Judé S, et al. Quantitative Gd-DOTA-based Aerosol Deposition in Asthmatic and Emphysematous Rats using UTE-MRI. 2015. Proceedings of the 23th Annual Meeting of ISMRM. p 3980.
4. Wang H, Willoquet G, Sebrié C, et al. Quantitative Aerosol Deposition in Mechanically-Ventilated Healthy and Asthmatic Rats using UTE-MRI. 2016; Singapore. Proceedings of the 24th Annual Meeting of ISMRM. p 1610.
5. Raza Asim MB, Shahzad M, Yang X, Sun Q, Zhang F, Han Y, Lu S. Suppressive effects of black seed oil on ovalbumin induced acute lung remodeling in E3 rats. Swiss medical weekly 2010;140:w13128.
6. Chong BT, Agrawal DK, Romero FA, Townley RG. Measurement of bronchoconstriction using whole-body plethysmograph: comparison of freely moving versus restrained guinea pigs. Journal of pharmacological and toxicological methods 1998;39:163-168.
7. Schabel MC, Parker DL. Uncertainty and bias in contrast concentration measurements using spoiled gradient echo pulse sequences. Physics in medicine and biology 2008;53:2345-2373.
8. Tzeng Y-S, Lutchen K, Albert M. The difference in ventilation heterogeneity between asthmatic and healthy subjects quantified using hyperpolarized 3He MRI. Journal of Applied Physiology 2009;106:813-822.
9. Rottier BL, Rubin BK. Asthma medication delivery: mists and myths. Paediatric respiratory reviews 2013;14:112-118; quiz 118, 137-118.
10. Katz I, Pichelin M, Montesantos S, et al. Using helium-oxygen to improve regional deposition of inhaled particles: mechanical principles. Journal of aerosol medicine and pulmonary drug delivery 2014;27:71-80.
Figures