4896
Asthma Ventilation Abnormalities Measured using Fourier-Decomposition Free-breathing Pulmonary 1H MRI1Robarts Research Institute, London, ON, Canada, 2Centre for Heart Lung Innovation, Vancouver, ON, Canada, 3Division of Respirology, University of Western Ontario, London, ON, Canada
Synopsis
Hyperpolarized noble-gas-MRI provides a way to visualize and regionally measure ventilation-heterogeneity in asthma, which has been shown to be sensitive to treatment response. Fourier-decomposition of free-breathing 1H MRI (FDMRI) has been proposed as an alternative way to evaluate regional-ventilation without the need for exogenous contrast. We hypothesized that ventilation-abnormalities would be qualitatively and quantitatively similar between the two imaging methods, and hence our objective was to measure ventilation-defects using FDMRI in asthma patients for comparison with inhaled-gas-MRI. Preliminary results in asthma showed that FDMRI ventilation-abnormalities were related to hyperpolarized noble-gas-MRI and clinical measurements of ventilation-heterogeneity in severe-asthmatics.
Purpose
Asthma is a chronic airways disease that is typically diagnosed and monitored using pulmonary-function-tests. Such measurements of airflow-limitation made at the mouth have been shown to be related to airway inflammation, bronchoconstriction, edema, mucus-hypersecretion, and plasma exudation.1 Although inexpensive and rapid, airflow-limitation measurements do not provide regional or mechanistic information that may provide a better understanding of the root causes of asthma symptoms and acute disease worsening needing hospital-based care. Recently, inhaled-gas MRI measurements of ventilation heterogeneity were evaluated in severe asthmatics and these were shown to explain asthma severity and exacerbations/control.2 These important and encouraging results are motivating the development of less complex 1H MRI acquisition and analysis methods for the measurement of pulmonary ventilation and perfusion. In this regard, Fourier-decomposition of free-breathing 1H MRI (FDMRI) has been proposed as a way to evaluate regional pulmonary ventilation by exploiting fast pulmonary MRI acquisition and non-rigid registration.3,4 Therefore, here our objective was to measure ventilation defects using FDMRI in asthma patients for comparison with hyperpolarized inhaled-noble-gas MRI pulmonary ventilation abnormalities. We hypothesized that by using both methods, ventilation abnormalities would be qualitatively and quantitatively indistinguishable.Methods
Pulmonary-Function-Tests:
Twenty participants with severe asthma (49±11yrs) provided written informed consent to an ethics-board-approved and Health Insurance Portability and Accountability Act compliant protocol. Plethysmography and spirometry were performed as previously described (MedGraphics-Corporation).5 Multiple-breath-nitrogen-washout (MBNW) was performed (ndd-Medical-Technologies) to measure the lung clearance index (LCI).6
Image-Acquisition:
Hyperpolarized 3He ventilation-images (total-acquisition-time=10s; TR/TE/flip-angle=3.8ms/1.0ms/7°; FOV=40×40cm2; matrix=128×80; BW=62.5kHz; NEX=1; number-of-slices=15; slice-thickness=15mm), 129Xe ventilation-images (total-acquisition-time=16s; TR/TE/flip-angle=7.0ms/1.8ms/variable; FOV=40×40cm; matrix=128×128; BW=9.0kHz; NEX=1; number-of-slices=16; slice-thickness=15mm), 1H anatomical-images (total-data-acquisition-time=16s; TR/TE/flip-angle=4.7ms/1.2ms/30°; FOV=40×40cm; matrix=128×80; BW=24.4kHz; NEX=1; number-of-slices=15; slice-thickness=15mm), and free-breathing 1H images (total-acquisition-time=125s; TR/TE/flip-angle=1.9ms/0.6ms/15°; FOV=40×40cm2; matrix=128×128; BW=250kHz; NEX=1; number-of-slices=1; slice-thickness=30mm, number-of-phases=500) were acquired as previously described7,8 on a 3T Discovery MR750 system (General-Electric-Health-Care).
Image-Analysis:
Non-rigid image-registration was performed using a modality-independent-neighbourhood-descriptor (MIND) deformable-registration technique,9 which employs a local image-descriptor as the similarity measurement and is optimized using Gaussian-Newton optimization with diffusion-regularization, to compensate for respiratory-motion. The reference image was chosen so that the corresponding lung volume was consistent with an inhaled-gas MRI ventilation image; deformable-registration was performed to maximize the geometric-similarity between images. Pulmonary-voxel intensities were aligned along a time-axis; Fourier-transforms were performed on the signal intensity oscillation pattern generated from the pulmonary-voxel intensities from co-registered free-breathing 1H images. For each voxel, the magnitude of the respiratory-rate (corresponding to the frequency of the first ventilation-harmonic) was determined and used to generate the FDMR ventilation image. Semi-automated segmentation was used to generate ventilation-defect-percent (VDP) for both inhaled-gas and FDMR ventilation images in order to identify ventilation abnormalities10 and their relationships with other clinical measurements.
Statistics:
Shapiro-Wilk tests were used to determine the normality of the data. Spearman correlation coefficients (ρ) were used to determine the relationship between MR ventilation measurements and the LCI. Bland-Altman analysis was implemented to determine the agreement between ventilation measurements. All statistics were performed using GraphPad Prism version 7.00 (GraphPad-Software-Inc) and results were considered significant when the probability of two-tailed type I error was less than 5% (p<.05).
Results
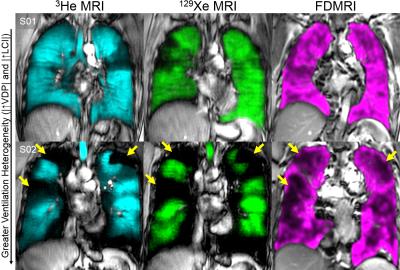
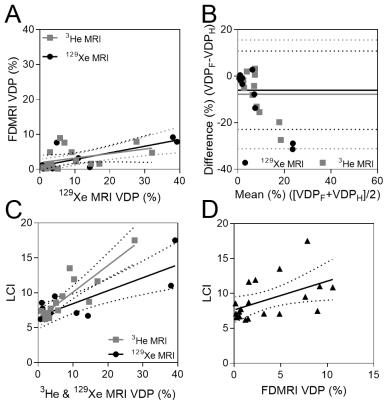
We consented and evaluated 20 asthmatics and their demographic information is provided in Table 1. Figure 1 shows hyperpolarized 3He, 129Xe and FDMRI centre coronal slice ventilation maps for representative asthmatics. There was qualitative spatial agreement for ventilation defects (ventilation heterogeneity including size and number of defects) for the subject with abnormal LCI -an independent measurement of ventilation heterogeneity. Figure 2 shows the relationships and agreement for MRI and LCI measurements. FDMRI was correlated with 3He MRI VDP (ρ=.61, p=.01) and 129Xe MRI VDP (ρ=.63, p=.04), although Bland-Altman analysis indicated that there were biases of -6.0±8.6% [-22.9%-10.8%] and -7.8±11.9% [31.2%-15.6%] for 3He and 129Xe, respectively. Furthermore, LCI was significantly correlated with FDMRI VDP (ρ=.54, p=.01), 3He VDP (ρ=.85, p<.01), but not 129Xe VDP (ρ=.36, p=.4).Discussion
In this proof-of-concept demonstration, we generated FDMRI in 20 participants with asthma to visualize and measure ventilation abnormalities. While FDMRI ventilation and perfusion imaging is currently limited to only a single lung slice and is inherently 2D, results showed that regional FDMRI ventilation abnormalities were spatially related to inhaled gas MRI ventilation defects. Moreover, FDMRI ventilation defects were also related to other clinical measurements of ventilation heterogeneity in severe asthmatics. Importantly, there were spatial differences and FDMRI ventilation defects are smaller than inhaled gas MRI venatilion defects.Conclusion
Overall, FDMR ventilation imaging is visually and quantitatively comparable to that of inhaled gas MR. These results are promising and driving our development of 3D whole-lung FDMRI over shorter acquisition times.Acknowledgements
We thank Trevor Szekeres, RTMR and Dave Reese, RTMR for MRI of research volunteers.References
1 Locksley, R. M. Asthma and allergic inflammation. Cell 140, 777-783, doi:10.1016/j.cell.2010.03.004 (2010).
2 Svenningsen, S., Nair, P., Guo, F., McCormack, D. G. & Parraga, G. Is ventilation heterogeneity related to asthma control? European Respiratory Journal, ERJ-00393-02016 (2016).
3 Bauman, G. et al. Non-contrast-enhanced perfusion and ventilation assessment of the human lung by means of fourier decomposition in proton MRI. Magnetic Resonance in Medicine 62, 656-664 (2009).
4 Deimling, M., Jellus, V., Geiger, B. & Chefd'Hotel, C. in Proceedings of the Sixteenth Meeting of the International Society for Magnetic Resonance in Medicine. Berkeley, CA: International Society for Magnetic Resonance in Medicine. 2639.
5 Miller, M. R. et al. Standardisation of spirometry. The European respiratory journal 26, 319-338, doi:10.1183/09031936.05.00034805 (2005).
6 Becklake, M. R. A new index of the intrapulmonary mixture of inspired air. Thorax 7, 111-116 (1952).
7 Capaldi, D. P. et al. Free-breathing Pulmonary (1)H and Hyperpolarized (3)He MRI: Comparison in COPD and Bronchiectasis. Academic radiology 22, 320-329, doi:10.1016/j.acra.2014.10.003 (2015).
8 Sheikh, K. et al. Magnetic resonance imaging biomarkers of chronic obstructive pulmonary disease prior to radiation therapy for non-small cell lung cancer. European journal of radiology open 2, 81-89, doi:10.1016/j.ejro.2015.05.003 (2015).
9 Heinrich, M. P. et al. MIND: Modality independent neighbourhood descriptor for multi-modal deformable registration. Medical image analysis 16, 1423-1435 (2012).
10 Kirby, M. et al. Hyperpolarized 3He magnetic resonance functional imaging semiautomated segmentation. Academic radiology 19, 141-152, doi:10.1016/j.acra.2011.10.007 (2012).
Figures