4893
T1 Mapping with Nitric Oxide Synthase (NOS) Inhibition Detects Impaired Coronary Endothelial Function in Mice Fed a High Fat Diet1Biomedical Engineering, University of Virginia, Charlottesville, VA, United States, 2Radiology, University of Virginia, Charlottesville, VA, United States
Synopsis
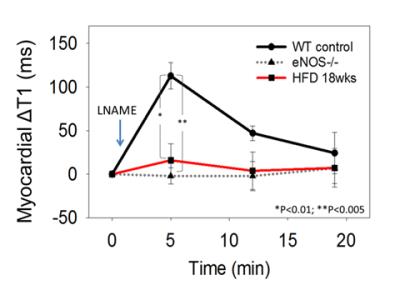
Endothelial nitric oxide synthase (eNOS)-mediated production of NO regulates the microvasculature, controlling both vessel diameter and permeability. We hypothesized that T1 mapping of the healthy heart during NOS inhibition would detect increased water content resulting from increased coronary microvascular permeability, while a blunted change in T1 between baseline and NOS inhibition would indicate coronary eNOS dysfunction. Using these methods, we detected an increase in myocardial T1 of 113±15 ms due to NOS inhibition in control mice, but no change in eNOS-/- or HFD mice, demonstrating the eNOS mechanism and detection of coronary endothelial dysfunction in a model of heart disease.
Introduction
Endothelial dysfunction occurs in many types of cardiovascular diseases. As an early and sensitive biomarker of disease, it has been called a “barometer” of cardiovascular health [1]. Endothelial nitric oxide synthase (eNOS)-mediated production of NO plays a central role in the process, controlling vessel diameter and maintaining low microvascular permeability. We hypothesized that native T1 mapping of the healthy heart and microvasculature during NOS inhibition would detect elevated myocardial T1 corresponding to increased interstitial water content resulting from increased microvascular permeability. In contrast, a blunted change in myocardial T1 between baseline and NOS inhibition would be indicative of coronary eNOS dysfunction. Given that mice fed a high fat diet (HFD) are a model of cardiomyopathy due to obesity and diabetes without atherosclerosis [2, 3], we further hypothesized that T1 mapping with NOS inhibition would detect coronary endothelial dysfunction in HFD mice.Methods
Wild type (WT) C57Bl/6 mice on standard chow diet (n = 6), eNOS-/- mice of C57Bl/6 genetic background (n = 7), and WT C57Bl/6 mice fed a HFD for 18 weeks (n = 10) underwent MRI studies using a 7T system (Clinscan, Bruker). During MRI, mice were anesthetized with 1.25% isoflurane and maintained at 36 ± 0.5°C using circulating warm water. After localizer imaging, T1 mapping was performed in a mid-ventricular short-axis slice at baseline and at 5, 12 and 20 minutes after intravenous injection of 4mg/kg LNAME, a NOS inhibitor that rapidly increases coronary microvascular permeability [4]. The T1-mapping method used fuzzy-clustering of spiral k-space data to ensure heart rate independence as previously described [5], and was accelerated using sparse sampling and compressed-sensing to reduce the scan time to 7 minutes. To exclude that changes in T1 might be due to vasodilatory effects, gadolinium-DTPA-enhanced first-pass perfusion MRI was performed in a separate imaging session in WT mice (n=6) at baseline and 5 minutes after IV injection of LNAME.Results
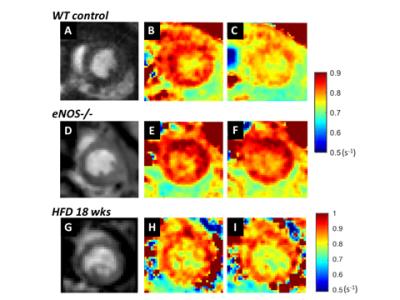
Figure 1 shows example R1 (1/T1) maps of the heart acquired before and 5 minutes after injection of LNAME in WT, eNOS-/- and HFD mice, demonstrating a decrease in R1 in response to NOS inhibition in WT mice, no decrease in eNOS-/- mice, and no decrease in R1 in HFD mice. Figure 2 summarizes data from all the mice and shows that NOS inhibition with LNAME causes a transient and significant increase in myocardial T1 in WT mice (ΔT1 = 113 ± 15 ms) but not in eNOS-/- or HFD mice. First pass MRI in WT mice measured similar perfusion at baseline (6.2 ± 0.4 ml/g/min) and 5 minutes after LNAME (6.1 ± 0.6 ml/g/min), suggesting no vasodilation.Conclusions
Inhibition of NOS with LNAME caused an increase in myocardial T1 in WT mice. As LNAME increases coronary microvascular permeability [4] and increases myocardial fluid flux [6] , the increased T1 is likely due to an increase in myocardial water content. The increase in myocardial T1 due to NOS inhibition was completely blunted in eNOS-/- mice, demonstrating that the LNAME-induced T1 increase is mediated through eNOS. Using these novel methods for assessing coronary eNOS function, we detected impaired coronary endothelial function in mice fed a HFD for 18 weeks, a time point where HFD mice are known to have impaired coronary microvascular function [3]. Future work will test the hypothesis that coronary endothelial dysfunction precedes the impairment of myocardial perfusion reserve in this model, providing an earlier biomarker of coronary microvascular disease with potential utility for evaluation of experimental therapies aimed at protecting the coronary microvasculature.Acknowledgements
Funding: NIH R01 EB001763 and AHA pre-doctoral fellowship 16PRE29750008.References
1. Vita, J.A. and J.F. Keaney, Jr., Endothelial function: a barometer for cardiovascular risk? Circulation, 2002. 106(6): p. 640-2.
2. Calligaris, S.D., et al., Mice long-term high-fat diet feeding recapitulates human cardiovascular alterations: an animal model to study the early phases of diabetic cardiomyopathy. PLoS One, 2013. 8(4): p. e60931.
3. Naresh, N.K., et al., Cardiovascular magnetic resonance detects the progression of impaired myocardial perfusion reserve and increased left-ventricular mass in mice fed a high-fat diet. J Cardiovasc Magn Reson, 2016. 18(1): p. 53.
4. Filep, J.G., E. Foldes-Filep, and P. Sirois, Nitric oxide modulates vascular permeability in the rat coronary circulation. Br J Pharmacol, 1993. 108(2): p. 323-6.
5. Vandsburger, M.H., et al., Improved arterial spin labeling after myocardial infarction in mice using cardiac and respiratory gated look-locker imaging with fuzzy C-means clustering. Magn Reson Med, 2010. 63(3): p. 648-57.
6. Kubes, P. and D.N. Granger, Nitric oxide modulates microvascular permeability. Am J Physiol, 1992. 262(2 Pt 2): p. H611-5.
Figures