4890
A spontaneous type 2 diabetic rhesus monkey model for cardiac MR study1West China Hospital, Sichuan University, Chengdu, People's Republic of China, 2Mallinckrodt Institute of Radiology, Washington University School of Medicine in St. Louis, 3West China HospitaL, Sichuan University, Chengdu
Synopsis
The aim of this study was to provide a human-like diabetic monkey model for cardiovascular research. Myocardial function and tissue characterizations were measured by CMR in 14 diabetic and 5 control monkeys. In addition to diastolic dysfunction, minor and moderate diffuse fibrosis was shown in cardiac T1, T1r, ECV, and non-contrast fibrosis index images in all the hearts of all diabetic monkeys, confirmed by histopathology finding in one diabetic monkey.
Introduction
Diabetes mellitus is an independent risk factor for abnormal cardiac remodeling (e.g., diabetic cardiomyopathy) and heart failure.1 At an early stage, diabetic cardiomyopathy is manifested by diastolic heart failure with preserved ejection fraction.2 Diffuse myocardial fibrosis and an increase in extracellular matrix have been suggested as potential mechanisms for diastolic dysfunction and increased LV stiffness.3 Nonhuman-primate diabetic model provides the best human-like model to investigate the mechanism and progression of diabetes associated cardiovascular diseases. The aims of our study were to demonstrate feasibility of this diabetic monkey model for cardiac assessment and associated Cardiac MR (CMR) tissue characterizations with established and newly developed CMR fibrosis imaging methods.Method
A total of 19 male rhesus (14.27years±2.22years, 10.1kg±1.98kg) monkeys were studied. Fourteen of them were spontaneous type 2 diabetes mellitus (T2DM, FPG>104mg/dl, HbA1c: 4.5-7.3%) based on findings from transthoracic echocardiography and blood biochemical tests. The other five controls (HC, FPG<90mg/dl) are matched in age, weight and gender. Blood biochemical examination was tested during the four-week acclimation. CMR protocol included: whole-heart cine, precontrast T1 mapping and T1ρ mapping at two different spin-locking frequencies, postcontrast T1 mapping, and late gadolinium enhancement (LGE) imaging. All images were acquired during a breath-hold time by turning off ventilation for 15-20 sec. Pre-T1, post-T1 and extracellular volume (ECV) values were acquired using software available in the MRI system. Myocardial fibrosis index (mFI), which is a new non-contrast myocardial fibrosis index calculated based on the dispersion characteristics of T1ρ, and absooute T1ρ values were calculated. Cardiac function was calculated using cine images. One heart was harvested from a diabetic monkey with diastolic dysfunction after imaging. Hematoxylin and eosin and Masson staining were performed to confirm diffuse myocardial fibrosis. Comparisons between diabetic and control groups were made using unpaired student T test or nonparametric Mann-Whitney U test where appropriate for continuous variables. Correlations were analyzed with the Pearson method.Result
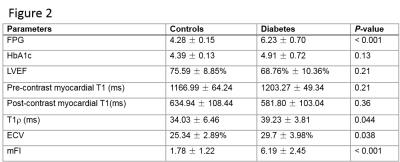
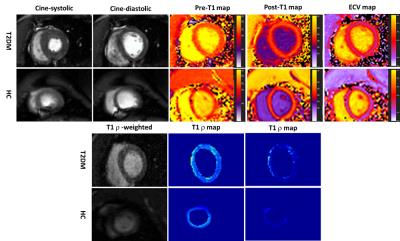
Echocardiographic results show all 14 monkeys in T2DM have diastolic dysfunction (8 monkeys in T2DM is E/A<1,E’/A’ >1; the other 6 in T2DM is E/A>1,E’/A’ <1), whereas E/A>1,E’/A’ >1 in all 5 controls. The blood biochemical and CMR parameters are demonstrated in Figure 1 for two groups of monkeys. Amoung then, the result of main parameters are showed as follow: global T1ρ values(B1=12µT)(T2DM, 39.23ms±3.81ms,Vs. HC,34.03ms±6.46ms P=0.044), global pECV (T2DM, 13.79±4.30,Vs. HC,4.61±3.10 P<0.001), global mFI (T2DM, 6.19±2.45,Vs. HC, 1.78±1.22 P<0.001)and global ECV (T2DM, 29.7%±3.98%,Vs. HC,25.34%±2.89% P=0.038)in T2DM is significantly higher than the controls respectively. Figure 2 shows CMR parametric maps in one diabetic monkey and one control monkey. Meanwhile, there was a positive correlation between mFI and ECV (r=0.46, P=0.046). For focal fibrosis, there was no evidence of regional late contrast enhancement in all animals. Histopathology showed that the myocardial extracellular space was widened with diffuse fibrosis with ECV = 37.46% and mFI =5.85 in this diabetic monkey.Discussion/ Conclusion
The spontaneous type 2 diabetes rhesus monkeys demonstrated similar characterizations of diabetic cardiomyopathy as in human. The minor to moderate diffuse fibrosis in diabetic monkeys was well assessed with both ECV and mFI, but not by pre- or post-contrast T1 mapping. More studies are needed to define the role of this monkey model for cardiac research in both diagnosis and therapeutical treatments.Acknowledgements
No acknowledgement found.References
1. Gottdiener JS, Arnold AM, Aurigemma GP, Polak JF, Tracy RP, Kitzman DW, et al. Predictors of congestive heart failure in the elderly: the Cardiovascular Health Study. J Am Coll Cardiol 2000;35(6):1628–37.
2. Rijzewijk LJ, van der Meer RW, Smit JW, Diamant M, Bax JJ, Hammer S, et al. Myocardial steatosis is an independent predictor of diastolic dysfunction in type 2 diabetes mellitus. J Am Coll Cardiol 2008;52(22):1793–9.
3. From AM, Scott CG, Chen HH. The development of heart failure in patients with diabetes mellitus and pre-clinical diastolic dysfunction: a population-based study. J Am Coll Cardiol 2010;55(4):300–5.