4889
Multiparametric CMR protocol and analysis methods for the detection of early cardiotoxicity remodelling in the mini-swine.1Mechanical Engineering, Polytechnique Montreal, Montreal, QC, Canada, 2Research Center, CHUM, Montreal, QC, Canada, 3Pediatric cardiology, CHU Sainte-Justine, Montreal, QC, Canada, 4Health Center, McGill University, Montreal, QC, Canada, 5Kinesiology, Université de Montréal, Montreal, QC, Canada
Synopsis
Some Cardiovascular magnetic resonance (CMR) studies investigated the long term effects of cancer treatments, but were never applied to the detection of early changes during cardiotoxicity remodelling. The CMR parameters we investigated in the miniature swine therapeutic model with doxorobucin was able discriminate treated animals from controls. Differences were detectible earlier than onset of classical echocardiographic changes. Translating these observations to personalized medicine approach could be the premise for the oncologist to know accurately when the treatment just starts to have deleterious effect on myocardium instead of just observing that the heart was damaged by doxorubicin.
Purpose
Cancer chemotherapy is an effective treatment to treat cancer in both adults and children. However, the associated cardiotoxicity of doxorubicin, the most commonly used anticancer agent, is a well-known serious side effect leading to long-term morbidity. Some Cardiovascular magnetic resonance (CMR) studies investigated the long term effects of cancer treatments (1,2,3), but were never applied to the detection of early cardiotoxicity remodelling so that preventive strategies can be applied and evaluated. We simulated doxorubicin-induced cardiotoxicity in the miniature swine and assessed the sensitivity of a novel CMR protocol and associated analysis methods to detect early cardiotoxicity remodelling.Methods
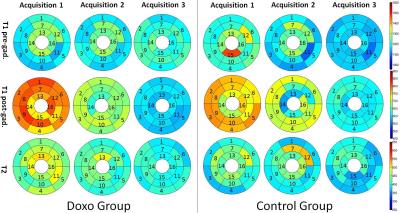
Female Yucatan miniature swine received either a chemotherapy treatment using doxorubicin (doxorubicin group, n=5, 5 doses of 75mg/m2, 3 weeks interval) or saline (control group, n=2). Three MRI scans and three echocardiography exams were performed before the 1st injection (A1), after the 4th injection (A2), and 3 weeks after the last injection (A3) (Figure 1). The CMR protocol consisted of an ECG-gated cine TruFISP sequence (slice thickness 6 mm, repetition time 37.2s, effective echo time 1.63s, flip angle 48°, matrix 174 × 208 and spatial resolution 1.05mm × 1.05mm), a MOLLI sequence for T1 mapping and a T2-prepared TrueFISP sequence for T2 mapping. Post-gadolinium T1 images were acquired using ProHance (0.2ml/kg, Bracco, USA) as a contrast agent. We developed new imaging analysis methods including a semi-automatic segmentation based on an interactive implementation of cubic Bezier curves and a finite element model with an isotropic hyperplastic behavior law and intra-myocardial displacements injected as loading conditions. T1 and T2 maps were generated using phase sensitive inversion recovery (PSIR) fitting and unsupervised curve-fitting based on a two-parameter equation respectively. Hematology lab results, clinical signs, echocardiography measures and CMR parameters were compared using a two‐ways analysis of variance, the first way being the group (doxorubicin versus control) and the second way being the acquisition (A1, A2, A3).Results
The miniature swine from the doxorubicin group developed alopecia, suffered from diarrhea and presented decreased white blood cells and platelets. In echocardiography, reductions of shortening fraction, ejection fraction and aortic blood flow were apparent at the last exam in the doxorubicin group. From cine-CMR, areas, lengths, thicknesses and LV torsion presented similar patterns along the cardiac cycle, but with different amplitudes between acquisitions and groups. For the LV end-diastolic cumulative displacements (Figure 2), the curve amplitude increased from A1 to A3 in the control group while it remains constant in the doxorubicin group. Cumulative von-Mises strain decreased at A3 in systole and late diastole in the doxorubicin group (Figure 3). T1pre decreased from A1 to A3 in the control group, T1post decreased from A1 to A3 in both control and doxorubicin groups, and T2 decreased from A1 to A3 in the doxorubicin group (Figure 4).Discussion
The decrease of ejection fraction and shortening fraction in our doxorubicin group observed in the last exam corresponds to the literature findings (1,4). The increase of the wall thicknesses and displacements from A1 to A3 in the control group could be explained by the normal growth of the heart. In the doxorubicin group, these parameters remained constant, suggesting that the animals did not grow as well as the control group, as confirmed by the reduced weight in the doxorubicin group. The decrease of ventricular area and length in the doxorubicin group also suggested these parameters as good indicators of the heart health, deteriorated by the doxorubicine. The slight reduction in T1pre measures in the control group from A1 to A3 could be associated to an increased lipid deposition, associated to the weight gain during the study. The decrease observed in T1post measures from A1 to A3, more important in the doxorubicin group than in the control group, might be related to myocardial diffuse fibrosis. The main limitation of the project was the small number of animals inducing a low statistical power. Thus, we focused on analytical analysis to understand the parameter behaviours.Conclusion
The CMR parameters we investigated in the miniature swine therapeutic model with doxorobucin was able discriminate treated animals from controls. Differences were detectible earlier than onset of classical echocardiographic changes. Translating these observations to personalized medicine approach could be the premise for the oncologist to know accurately when the treatment just starts to have deleterious effect on myocardium instead of just observing that the heart was damaged by doxorubicin.Acknowledgements
We thank the CRCHUM’s Animal facility for the treatment and care of the animals.This project was funded by the “Fonds de Recherche du Québec en Nature et Technologies” (FRQNT, Team grant) and the Natural Sciences and Engineering Research Council of Canada (NSERC, Discovery grant).
References
1. K. Ylanen, T. Poutanen, P. Savikurki-Heikkila, I. Rinta-Kiikka, A. Eerola, and K. Vettenranta, "Cardiac magnetic resonance imaging in the evaluation of the late effects of anthracyclines among long-term survivors of childhood cancer," J Am Coll Cardiol, vol. 61, pp. 1539-47, Apr 9 2013. 2. Armstrong GT, Plana JC, Zhang N, Srivastava D, Green DM, Ness KK, Daniel Donovan F, Metzger ML, Arevalo A, Durand JB, Joshi V, Hudson MM, Robison LL, Flamm SD. Screening adult survivors of childhood cancer for cardiomyopathy: comparison of echocardiography and cardiac magnetic resonance imaging. J Clin Oncol. 2012 : 30(23):2876-84. 3. Tham EB, Haykowsky MJ, Chow K, Spavor M, Kaneko S, Khoo NS, et al. Diffuse myocardial fibrosis by T1-mapping in children with subclinical anthracycline cardiotoxicity: relationship to exercise capacity, cumulative dose and remodeling. J Cardiovasc Magn Reson 2013, 15:48. 4. Christiansen S, Perez-Bouza A, Schalte G, Hilgers RD, and Autschbach R. Selective left ventricular adriamycin-induced cardiomyopathy in the pig," J Heart Lung Transplant 2008, 27:86-92.Figures