4886
4D Cine Strategy for Assessment of Mouse Cardiac Function and Infarct Size in a Single Acquisition Optimized for a Clinical 3T MR System1Department of Radiology, Geneva University Hospitals, Geneva, Switzerland, 2Department of Internal Medicine, University of Genoa, Italy, 3Department of Cardiology, Geneva University Hospitals, Geneva, Switzerland
Synopsis
Small cardiac imaging on clinical 3T machines is an important, cost effective translational step for new contrast media and sequence validation. We developed for the first time a new 4D strategy tailored for 3T to simultaneously assess function and infarct in mice, validated against 2D cine, post mortem and histology. Isotropic 3D cardiac cine of mice on a clinical 3T system improved coverage and reduced flow artifacts with higher spatial and temporal resolution for more accurate quantification of cardiac function. Infarct volume enhancement was quantifiable from the same 3D cine acquisition.
Background
We developed a single acquisition 4D strategy to simultaneously assess function and infarct in mice utilizing improved spatial and temporal resolution over 2D cine. The acquisition was tailored for imaging on a clinical 3T. Small animal cardiac imaging on clinical machines is an important, cost-effective, translational step for new contrast media and sequence development, but suffers hardware limitations compared to dedicated systems.Materials and Methods
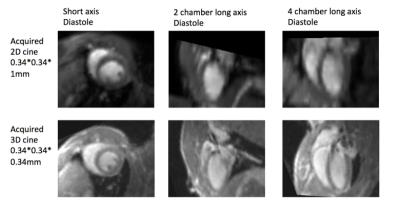
Adult C57BL/6J mice underwent 60 minutes ischemia followed by reperfusion. Controls had no surgery. Groups consisted of n=14 infarct and 4 control for function; and 6 infarct and 6 control for histology/reproducibility. MRI was 24hrs after surgery, with gadolinium (Gd) injection, and sacrifice at the end of the in vivo imaging for post-mortem MRI and histology. The 3D GRE cine sequence (ECG/respiratory triggered) had isotropic resolution 344μm, TR/TE 7.8/2.9ms, acquisition time 25-35 minutes. The conventional 2D FLASH cine sequence had the same in-plane resolution 344μm, slice thickness 1mm, TR/TE 11/5.4ms, acquisition 20-25 minutes (+ 5-10 minutes for slice planning). Left ventricle (LV) and right ventricle (RV) volumes were measured and statistical comparison was made between 2D and 3D along with inter/intra-observer reproducibility. The physiological equality for left and right ejection volume was verified. MRI infarct volume was compared to histology1 and function.Results
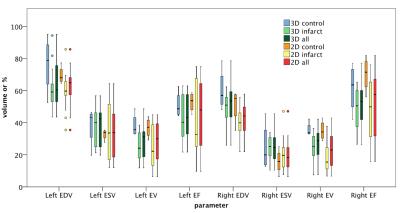
The ‘4D strategy’ of 3D cine (3D) MRI outperformed 2D cine (2D) for spatial and temporal resolution. 3D required no complex planning. Flow artifacts were significantly reduced in 3D (p=0.008) due to the shorter TE. CNR allowed epi/endocardial delineation for 2D and 3D with ICC intra/inter reproducibility of 0.2-0.99 for 2D and 0.6-0.99 for 3D. 3D isotropic coverage simplified/improved apex and right ventricle (RV) assessment. All 3D left volumes and right ejection fraction showed no difference to 2D (p>0.05). Grouping all mice, significant correlation 2D to 3D was observed for all parameters with R2 from 0.34 to 0.67. Ejection volume was equal for LV and RV as expected for mouse anatomy, therefore acting as internal validation. LV mass correlated 2D to 3D and 3D RV mass correlated with postmortem (R2>0.70) but was not measurable on 2D.
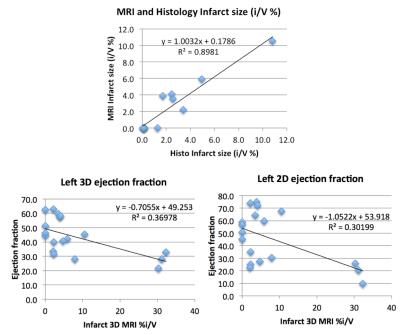
‘Late Gd enhancement’ was seen clearly only with 3D and this quantifiable infarct volume correlated to histology (R2=0.89) with both small transmural infarcts (ligature site) and large nontransmural infarcts covering the whole apex. Left ejection fraction and MRI-measured infarct volume correlated (R2>0.3).
Discussion
On the 3D cine, it is possible to visualize and contour correctly more basal slices due to higher, isotropic spatial resolution. Partial volume effects of the thicker slices in 2D means that the definition of smaller structure at the base of the RV is lost and the segmentation does not include the final slices comparable to 3D. Flow artifacts obscure the valve level in the 2D cine but are reduced on 3D due to shorter TE along with better temporal resolution for defining systole. With the thinner slices in 3D it was also easier to accurately include the right ventricular outflow tract. This gives more accurate values for 3D, but could also lead to a difference for the functional parameters between 2D to 3D, due to the partial or complete inclusion of the right ventricular outflow tract in 2D contouring. Improved spatial resolution in 3D allowed better RV assessment bringing advantages in cardiac mouse MRI and certain pathologies that would benefit from more accurate assessment of RV function.
3D cine with a shorter TR (7.8ms c.f. 11ms in 2D, or 18 c.f. 13 cardiac phases) yields similar temporal improvements to previously reported regularization for 2D cine but with a much higher spatial resolution 2. Despite a lack of gold standard for ejection volumes, left-to-right correlation for 3D ejection volumes, as is expected in mice, agrees with physiological state and confirms accuracy of our 3D method. For 2D, as well as time-consuming localization preparation scans, there is the possibility of misregistration between short axes acquired in separate scans, as opposed to a single 3D acquisition. This misregistration would also exist between the 2D cine and any 2D Gd enhanced acquisitions for infarct assessment.
Conclusion
In conclusion, we developed a 4D cardiac strategy after contrast media injection in mice. Function and infarct were imaged simultaneously and assessed using internal/physiological validation and comparison to conventional cine images and histology. This 4D strategy was faster, easier to use and, due to isotropic resolution, has strong potential for both LV and RV volume, function and mass assessment in mice on clinical systems. The added advantage is the simultaneous infarct volume quantification meaning the 4D strategy could replace separate 2D function and infarct MRI techniques.Acknowledgements
No acknowledgement found.References
1 Braunersreuther V, et al. (2013). Thromb Haemost 110:807-825.
2 Delattre BM, et al. (2012). IEEE Trans Biomed Eng 59: 929-935.
Figures