4884
Cardiac MRI measurement of right ventricular strain using feature tracking in a model of embolic pulmonary hypertension.1Radiology, University of Wisconsin-Madison, Madison, WI, United States, 2Radiology and Medical Engineering, University of Wisconsin-Madison, Madison, WI, United States, 3Veterinary Medicine, University of Wisconsin-Madison, Madison, WI, United States, 4Biomedical Engineering, University of Wisconsin-Madison, Madison, WI, United States
Synopsis
Right ventricular strain was assessed using an MRI tissue tracking algorithm on bSSFP axial sequences in both acute and chronic embolic pulmonary hypertension canine models. Strain values were heterogeneous in the acute population with statistically significant decreases in acute radial and longitudinal strain rate and chronic radial and longitudinal strain and strain rate values. Findings suggest MRI cardiac strain measurement is a promising technique in the clinical evaluation of post embolic pulmonary hypertension patients.
Purpose
Evaluate changes in right ventricular (RV) strain using a tissue-tracking algorithm in acute and chronic models of embolic pulmonary hypertension (AEPH and CEPH, respectively).Background
Pulmonary embolism is a common pathologic condition which may cause pulmonary hypertension (PH) and subsequent right ventricular (RV) dysfunction. The degree of RV dysfunction has been shown to be an important prognostic indicator of patient morbidity[1]. Evaluation of RV function through the measurement of cardiac strain has been prompted by both a need to evaluate the function of the morphologically asymmetric RV, which is difficult with echocardiography, and data indicating strain can be an early and cardiac load independent indicator of function within the left ventricle[2,3]. Cardiac magnetic resonance (CMR) imaging has long offered the ability to measure RV function through quantitative measurement of ejection fraction and qualitative assessment of cardiac strain through dedicated tagged myocardial sequences. New tissue tracking algorithms now offer the ability to retrospectively measure cardiac strain on standard cine balanced steady-state free precession (bSSFP) sequences with additional benefits of increased signal to noise ratio and lack of requirement for imaging windows and assumptions of symmetry when compared to echocardiography[4].Methods
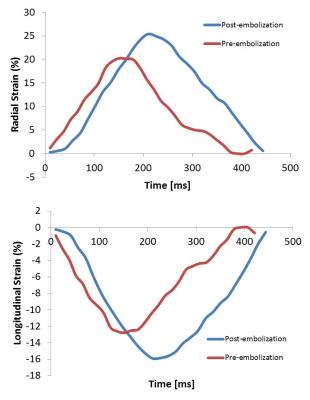
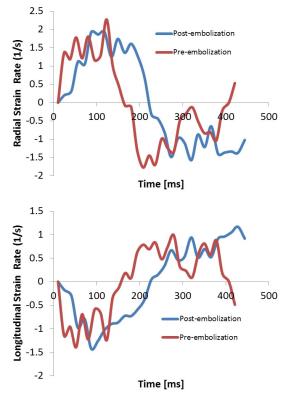
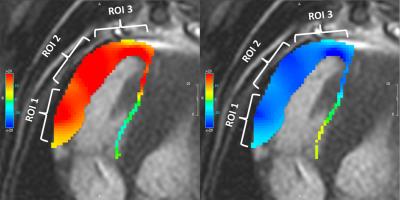
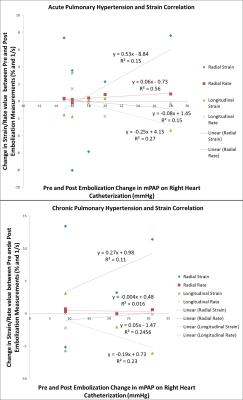
Following an IACUC approved protocol, cardiac magnetic resonance (CMR) and right heart catheterization (RHC) was performed in 11 canines preceding and following pulmonary microbead (150-500µm) embolization. CMR, consisting of axial cine bSSFP images acquired through the entire RV, was performed on a 3.0T MR scanner (MR 750, GE Healthcare, Waukesha, WI). Post-embolization RHC and CMR were performed immediately after induction of acute PH in six canines and after induction of chronic embolic PH in 4 canines with one CEPH canine excluded from MR following failure to develop PH. Baseline and post-embolization radial and longitudinal strain and strain rates were measured using a tissue-tracking algorithm (cmr42, Circle Cardiovascular Imaging, Inc., Calgary, Canada) (Figure 1, 2). Evaluation was limited to the RV free wall which was subdivided into three segments (apical, mid and base) (Figure 3). Pre- and post-embolic PH strain measurements were compared using a paired, two-tailed t-test. A scatter plot was used to perform regression analysis between changes in RV strain and changes in pulmonary arterial pressure measurements.Results
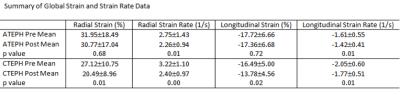
Pre and post embolization AEPH mPAP measured 16.5 ±3.1 mmHg and 34.25 ±12.1 mmHg respectively (p<0.05) with CEPH pre and post embolization mPAP measuring 14.2 ±3.5 mmHg and 33±7.6 mmHg respectively (p<0.05). A summary of right ventricular free wall strain and strain rate data is depicted in Table 1. Statistically significant change was noted in the AEPH strain rate measurements and CEPH strain and strain rate measurements. Segmental analysis demonstrated statistical significance in AEPH segment 1 radial strain rate (p<0.05). The CEPH model demonstrated statistically significant change in radial and longitudinal segment 1 strain rates with radial strain and strain rates statistically significant in segment 2 (p<0.05). Scatter plots comparing change in strain and change in mPAP demonstrated no correlation to modest correlation (R2 = 0.016 to 0.56) (Figure 4).Discussion
In this study, we observed differences in the effects of acute and chronic embolic PH on RV strain and strain rates. In acute embolic PH, heterogeneous results were obtained for strain values and only radial and longitudinal strain rate significantly changed globally. This is distinct from the changes that occurred in chronic embolic PH, where both radial and longitudinal global strain and strain rate significantly decreased compared to baseline. Furthermore, statistically significant change was observed at the segmental level across all types of strain measurement in the CEPH model. The changes in both the AEPH and CEPH models were not well correlated with change in mPAP from right heart catheterization.Conclusion
Overall, cardiac strain proved to be a viable metric in determining changes in RV function in both AEPH and CEPH models with differences between the two models possibly explained by evolving strain values over time. These findings suggest cardiac strain would be a valuable addition to CMR particularly in evaluating patients with pulmonary embolism or alternate etiology of pulmonary hypertension as RV function is an important prognostic indicator. Further study of cardiac strain in a pulmonary hypertensive human population is warranted.Acknowledgements
University of Wisconsin-Madison Radiology Department R&D.
NIH R01HL105598-04 & R01HL086939
References
1. Peacock AJ, Crawley S, McLure L, et al. Changes in Right Ventricular Function Measured by Cardiac Magnetic Resonance Imaging in Patients Receiving Pulmonary Arterial Hypertension–Targeted Therapy. Circ Cardiovasc Imaging. 2014;7(1).
2. da Costa Junior AA, Ota-Arakaki JS, Ramos RP, et al. Diagnostic and prognostic value of right ventricular strain in patients with pulmonary arterial hypertension and relatively preserved functional capacity studied with echocardiography and magnetic resonance. Int J Cardiovasc Imaging. August 2016:1-8. doi:10.1007/s10554-016-0966-1.
3. Shah AM, Solomon SD. Myocardial Deformation Imaging. Circulation. 2012;125(2).
4. Pedrizzetti G, Claus P, Kilner PJ, et al. Principles of cardiovascular magnetic resonance feature tracking and echocardiographic speckle tracking for informed clinical use. J Cardiovasc Magn Reson. 2016;18(1):51. doi:10.1186/s12968-016-0269-7.
Figures