4883
Right ventricular myocardial strain in rats1Institute for Experimental Medical Research, University of Oslo, OSLO, Norway, 2Bjørknes College, Oslo, Norway
Synopsis
The function of the right ventricle (RV) is closely linked to clinical outcome in many cardiovascular diseases. Experimental heart disease in rodents play an irreplaceable role in modern cardiovascular research, but no in vivo method exists offering robust measurements of RV myocardial function in small animals.
We used phase-contrast MRI to measure RV strain in rats. We found that RV strain and ejection fraction were closely related, and confirmed that high RV afterload is linked to reduced RV strain.
We show, for the first time, that it is possible to accurately measure myocardial function in the RV in rodents.
Purpose
Recent technological advances have resulted in increased interest in the right ventricle (RV) with emphasis on its importance in many cardiac diseases.1-4 However, RV structure and function is difficult to study in vivo, and details of its role in heart disease remain largely unknown. The difficulties of accurately evaluating RV function in vivo are especially linked to its complex anatomy, thin wall and intricate contraction pattern.5, 6
Experimental heart failure in rodents offers, in combination with noninvasive imaging methods, unparalleled opportunities to perform longitudinal studies on disease development and the effect of intervention. But no technique has yet demonstrated an ability to measure in vivo RV myocardial function in rodents robustly.
In the left ventricle, phase-contrast MRI (PC-MRI) allow measurement of strain in rats with high temporal and spatial resolution.7 The purpose of the present study was to establish a PC-MRI method for measuring RV strain in rats and, as a proof-of-concept, apply the method in rats with different degree of RV afterload.
Methods
39 Wistar rats with left ventricular myocardial infarcts of varying size, plus 10 control animals, were examined six weeks after operation. At this time point, the rats exhibited varying degree of RV afterload.8
The PC-MRI experiments were performed on a 9.4T magnet (Agilent Technologies, Inc., USA) (see details in Table 1).9 A mid-RV short-axis slice and a mid-septal long-axis slice were acquired (Figure 1). The images were semi-automatically segmented10 and pixel-wise motion paths were calculated.11 The motion of the RV myocardium was tracked in segments, and longitudinal and circumferential strains and systolic strain rates were calculated from the relative motion of the segments (Figure 1). For comparison, tricuspid annular plane systolic excursion (TAPSE) was found from the longitudinal motion of the basal free-wall RV.
RV end-diastolic volume (RVEDV) and ejection fraction (RVEF) were measured using whole-heart cine MRI, left ventricular end-diastolic pressure (LVEDP) was measured by catheterization as an index of RV afterload, and the lungs were excised post-mortem and weighed. The MI rats were divided into low-afterload (LVEDP<15mmHg) and high-afterload (LVEDP>=15mmHg) groups.
All analysis was done using Matlab (The MathWorks, USA) using Kruskal-Wallis ANOVA and Spearman’s rho. Results are reported with median [interquartile range].
Results
In the high-afterload group, RVEDV and lung weight were higher and RVEF was lower than both other groups (Table 2). Peak free-wall longitudinal RV strain and strain rate, as well as peak free-wall circumferential strain rate, were reduced compared to the other two groups.
Group-independent correlations (Table 3) revealed that all free-wall parameters were correlated to RVEF and LVEDP. Of all parameter investigated, free-wall longitudinal peak strain and systolic strain rate were most closely related to elevated RV afterload.
Discussion
It is well established that RV function is sensitive to elevation in afterload,6 and we found that all PC-MRI parameters reflecting RV longitudinal function were reduced in the group with high RV afterload. In line with previous results in humans, we found that RVEF correlated with RV strain12-14 and that RV free-wall longitudinal strain was more closely related to RV afterload than TAPSE.14-16 Our data also indicate that circumferential strain was more closely affected by dilatation than longitudinal strain. This might be understood in light of change in geometry; as short-axis curvature is higher than long-axis curvature, and RV dilatation makes the ventricle more spherical, the strain produced by a given force is reduced.17
Speckle tracking echocardiography is a widely applied method for strain measurements in rodents, but the reproducibility and diagnostic utility of preclinical RV echocardiography is very limited18 and has not been shown to be able to measure RV strain in rats robustly19. MR feature tracking has been used to assess RV strain in humans1,20,21 but has been shown to be less accurate than PC-MRI in direct comparison.22
Importantly, better methods for quantitative assessment of the RV are highly desirable,23 both preclinically and clinically. Due to the inherent similarities between preclinical and clinical MRI, we expect that the method presented here is advantageous clinically as well, but this warrants further investigation.
Conclusion
We show, for the first time, that RV myocardial strain can be measured accurately in rats. We used PC-MRI to measure TAPSE, strain and strain rate in the RV free-wall and intraventricular septum in animals with different levels of RV afterload, and confirmed that RV strain is reduced when RV afterload is high.Acknowledgements
No acknowledgement found.References
1. Heermann P, Hedderich DM, Paul M, Schulke C, Kroeger JR, Baessler B, Wichter T, Maintz D, Waltenberger J, Heindel W, Bunck AC. Biventricular myocardial strain analysis in patients with arrhythmogenic right ventricular cardiomyopathy (ARVC) using cardiovascular magnetic resonance feature tracking. J Cardiovasc Magn Reson 2014;16:75.
2. Merlo M, Gobbo M, Stolfo D, Losurdo P, Ramani F, Barbati G, Pivetta A, Di LA, Anzini M, Gigli M, Pinamonti B, Sinagra G. The Prognostic Impact of the Evolution of RV Function in Idiopathic DCM. JACC Cardiovasc Imaging 2016.
3. Zornoff LA, Skali H, Pfeffer MA, St John SM, Rouleau JL, Lamas GA, Plappert T, Rouleau JR, Moye LA, Lewis SJ, Braunwald E, Solomon SD. Right ventricular dysfunction and risk of heart failure and mortality after myocardial infarction. J Am Coll Cardiol 2002;39:1450-1455.
4. Antoni ML, Scherptong RW, Atary JZ, Boersma E, Holman ER, van der Wall EE, Schalij MJ, Bax JJ. Prognostic value of right ventricular function in patients after acute myocardial infarction treated with primary percutaneous coronary intervention. Circ Cardiovasc Imaging 2010;3:264-271.
5. Sievers B, Addo M, Franken U, Trappe HJ. Right ventricular wall motion abnormalities found in healthy subjects by cardiovascular magnetic resonance imaging and characterized with a new segmental model. J Cardiovasc Magn Reson 2004;6:601-608.
6. Haddad F, Hunt SA, Rosenthal DN, Murphy DJ. Right ventricular function in cardiovascular disease, part I: Anatomy, physiology, aging, and functional assessment of the right ventricle. Circulation 2008;117:1436-1448.
7. Espe EK, Aronsen JM, Skardal K, Schneider JE, Zhang L, Sjaastad I. Novel insight into the detailed myocardial motion and deformation of the rodent heart using high-resolution phase contrast cardiovascular magnetic resonance. J Cardiovasc Magn Reson 2013;15:82.
8. Sjaastad I, Sejersted OM, Ilebekk A, Bjornerheim R. Echocardiographic criteria for detection of postinfarction congestive heart failure in rats. J Appl Physiol 2000;89:1445-1454.
9. Espe EK, Aronsen JM, Skrbic B, Skulberg VM, Schneider JE, Sejersted OM, Zhang L, Sjaastad I. Improved MR phase-contrast velocimetry using a novel nine-point balanced motion-encoding scheme with increased robustness to eddy current effects. Magn Reson Med 2013;69:48-61.
10. Espe EK, Skardal K, Aronsen JM, Zhang L, Sjaastad I. A semiautomatic method for rapid segmentation of velocity-encoded myocardial magnetic resonance imaging data. Magn Reson Med 2016.
11. Pelc NJ, Drangova M, Pelc LR, Zhu Y, Noll DC, Bowman BS, Herfkens RJ. Tracking of cyclic motion with phase-contrast cine MR velocity data. J Magn Reson Imaging 1995;5:339-345.
12. Focardi M, Cameli M, Carbone SF, Massoni A, De VR, Lisi M, Mondillo S. Traditional and innovative echocardiographic parameters for the analysis of right ventricular performance in comparison with cardiac magnetic resonance. Eur Heart J Cardiovasc Imaging 2015;16:47-52.
13. Yang LT, Yamashita E, Nagata Y, Kado Y, Oshima S, Otsuji Y, Takeuchi M. Prognostic value of biventricular mechanical parameters assessed using cardiac magnetic resonance feature-tracking analysis to predict future cardiac events. J Magn Reson Imaging 2016.
14. Arenja N, Riffel JH, Djiokou CN, Andre F, Fritz T, Halder M, Zelniker T, Kristen AV, Korosoglou G, Katus HA, Buss SJ. Right ventricular long axis strain-validation of a novel parameter in non-ischemic dilated cardiomyopathy using standard cardiac magnetic resonance imaging. Eur J Radiol 2016;85:1322-1328.
15. Focardi M, Cameli M, Carbone SF, Massoni A, De VR, Lisi M, Mondillo S. Traditional and innovative echocardiographic parameters for the analysis of right ventricular performance in comparison with cardiac magnetic resonance. Eur Heart J Cardiovasc Imaging 2015;16:47-52.
16. Lisi M, Cameli M, Righini FM, Malandrino A, Tacchini D, Focardi M, Tsioulpas C, Bernazzali S, Tanganelli P, Maccherini M, Mondillo S, Henein MY. RV Longitudinal Deformation Correlates With Myocardial Fibrosis in Patients With End-Stage Heart Failure. JACC Cardiovasc Imaging 2015;8:514-522.
17. Espe EK, Aronsen JM, Eriksen GS, Zhang L, Smiseth OA, Edvardsen T, Sjaastad I, Eriksen M. Assessment of regional myocardial work in rats. Circ Cardiovasc Imaging 2015;8.
18. Vildbrad MD, Andersen A, Andersen TK, Axelgaard S, Holmboe S, Andersen S, Ringgaard S, Nielsen-Kudsk JE. Limitations and pitfalls in measurements of right ventricular stroke volume in an animal model of right heart failure. Physiol Meas 2015;36:925-937.
19. Kimura K, Daimon M, Morita H, Kawata T, Nakao T, Okano T, Lee SL, Takenaka K, Nagai R, Yatomi Y, Komuro I. Evaluation of right ventricle by speckle tracking and conventional echocardiography in rats with right ventricular heart failure. Int Heart J 2015;56:349-353.
20. Prati G, Vitrella G, Allocca G, Muser D, Buttignoni SC, Piccoli G, Morocutti G, Delise P, Pinamonti B, Proclemer A, Sinagra G, Nucifora G. Right Ventricular Strain and Dyssynchrony Assessment in Arrhythmogenic Right Ventricular Cardiomyopathy: Cardiac Magnetic Resonance Feature-Tracking Study. Circ Cardiovasc Imaging 2015;8:e003647.
21. Vigneault DM, Te Riele AS, James CA, Zimmerman SL, Selwaness M, Murray B, Tichnell C, Tee M, Noble JA, Calkins H, Tandri H, Bluemke DA. Right ventricular strain by MR quantitatively identifies regional dysfunction in patients with arrhythmogenic right ventricular cardiomyopathy. J Magn Reson Imaging 2016;43:1132-1139.
22. Kuetting DL, Sprinkart AM, Dabir D, Schild HH, Thomas DK. Assessment of cardiac dyssynchrony by cardiac MR: A comparison of velocity encoding and feature tracking analysis. J Magn Reson Imaging 2016;43:940-946.
23. Popovic ZB, Min D. RV Function: Trying to Listen to Second Fiddle. JACC Cardiovasc Imaging 2016.
Figures
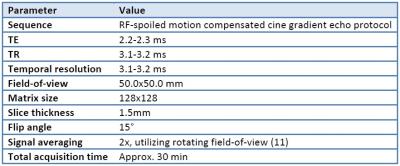
Table 1: PC-MRI acquisition parameters

Figure 1: Slice planning and strain measurements
Overview of the slice planning of short-axis (A) and long-axis (B) slices. Circumferential (C) and longitudinal (D) strains are found from the relative motion of neighboring segments along the myocardium. Panels E and F show a control heart in early systole and diastole, with overlaid strain measurements.
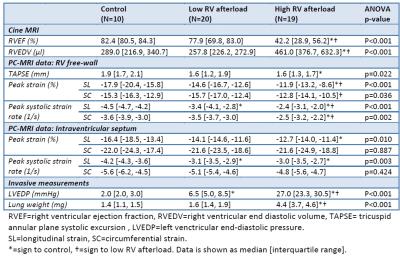
Table 2: MRI results in groups
RVEF=right ventricular ejection fraction, RVEDV=right ventricular end diastolic volume, TAPSE= tricuspid annular plane systolic excursion , LVEDP=left venctricular end-diastolic pressure. SL=longitudinal strain, SC=circumferential strain. *=sign to control, †=sign to low RV afterload. Data is shown as median [interquartile range].
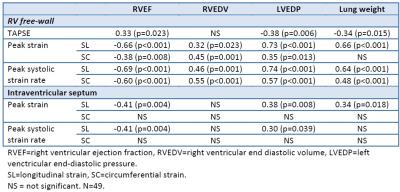
Table 3: Correlation analysis
RVEF=right ventricular ejection fraction, RVEDV=right ventricular end diastolic volume, LVEDP=left venctricular end-diastolic pressure. SL=longitudinal strain, SC=circumferential strain. NS = not significant. N=49.