4882
Quantitative gated and non-gated rat phase contrast MRI: optimized analysis of blood flow and wall shear stress.1Department of Biomedical Imaging and Radiological Science, China Medical University, Taichung, Taiwan, 2Center for Advanced Molecular Imaging and Translation, Chang Gung Memorial Hospital
Synopsis
Goals of this study are to test effects of gated/non-gated, velocity encoding (VENC) and spatial resolution on blood flow, wall shear stress (WSS) and artery area when performing phase-contrast (PC) MRI for rat common carotid artery (CCA). Results show the usage of gated instrument can provide more reproducible results. VENC has insignificant influences on flow, WSS and artery area. To compromise the trade-off between accuracy and time-consuming, the resolution of 0.21 mm is suggested for extracting hemodynamic information about rat CCA.
Purpose
Time-resolved phase-contrast magnetic resonance imaging (PC-MRI) has been a powerful means to provide insights into hemodynamic characteristics of blood flow. By using animal models, hemodynamic parameters from PC-MRI such as blood flow volume and wall sheer stress (WSS) distribution, can benefit the comparisons between normal and pathological models1. Therefore, parameter settings in rodent PC-MRI is crucial. Prior human studies have revealed blood flow measurements were not affected by the use of electrocardiogram (ECG)-gated implementations or not2. Taking considerable differences in scan duration into account, non-gated PC-MRI has gained its increasing popularity in human PC-MRI studies3, 4. For rat PC-MRI studies, however, vessel diameters are relative small and their hemodynamic features are significantly different when compared to those of human. Therefore, the central goal of this study is to test whether non-gated PC-MRI is appropriate for rat PC-MRI studies or not. Moreover, velocity encoding (VENC) selection and spatial resolution setting are link to the precise quantification. The blood flow, WSS and artery area from rat common carotid artery (CCA) measured with different VENC and spatial resolution values were discussed in this studies as well.Methods
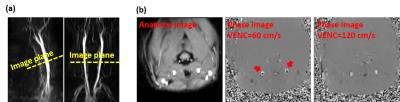
This study was approved by the local institutional animal ethics committee. A total of 20 Sprague Dawley (SD) rats (weight: 300-400 g) were scanned on a 7T MRI scanner (Bruker) and anesthetized with isoflurane throughout experiments. Study 1: Effects of gated/non-gated sequences and VENC on flow and WSS measurements. A 3D time-of-flight (TOF) angiogram was first performed to visualize the CCA. The image plane was targeted at the middle of CCA (Figure 1a). Next, gated and non-gated implementations were compared to determine the optimal sequence for PC-MRI in 12 rats. For each strategy, VENC of 60, 70, 80, 90, 100, 110 and 120 cm/s were scanned in randomized order and the numbers of repetition were 2 for each VENC setting to estimate the coefficient of variation (CoV). Any velocity aliasing due to the underestimated VENC parameters were corrected in post-processing5. With the VENC of 120 cm/s, no phase aliasing was observed in all rats (Figure 1b). Other imaging parameters are: single slice, FOV=40x40x2 mm, matrix size=128x128, number of average=8. Study 2: Effect of spatial resolution on flow and WSS measurements. To evaluate variations caused by different in-plane resolution, resolution values of 0.63, 0.31, 0.21 and 0.16 (the corresponding matrix size were respectively 64x64, 128x128, 192x192 and 256x256 at a given FOV) were assessed in 8 rats. The optimal parameters from Study 1 was employed.Results and Discussions
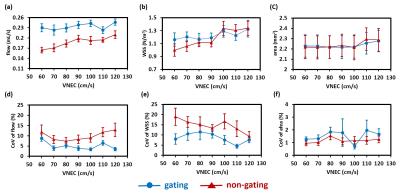
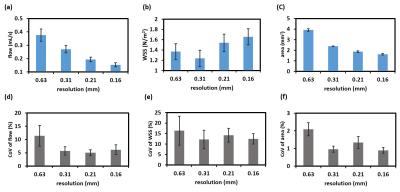
Study 1: Results of gated/non-gated strategies and varied values of VENC are displayed in Figure 2. Two way ANOVA with repeated measurement shows that, the usage of the gated sequence has a significant impact on flow (P<0.001), but not on WSS (P=0.39) and artery area (0.92). Moreover, the gated sequence demonstrates the smaller CoV in flow (P<0.001) and WSS (P<0.001) when compared to the non-gated one, suggesting the gated sequence has the leverage in test-retest reproducibility. With the help of the gated sequence, the values of flow, WSS, artery area, and their corresponding CoV values are independent of values of VENC (all P>0.05). Although the gated sequence sacrifices the scan time, it provides more precise measurements. We therefore recommend gated sequence with VENC of 120 cm/s could be employed for the following rat PC-MRI studies. Study 2: Phase images with different values of in-plane resolution from a representative rat are demonstrated in Figure 3. The boundary of artery becomes resolved when the better resolution is employed. Figure 4 shows the effect of resolution on flow, WSS and artery area. Analyzed by one way ANOVA with repeated measurement, the results show that, the in-plane resolution has significant influences on flow (P<0.001) and artery area (P<0.001), and is toward a trend for WSS (P=0.07). The lower resolution such as 0.63 mm tends to overestimate the flow and artery area. But when the resolution is 0.21 mm, the resulted flow and artery area were comparable to those obtained with the finest resolution of 0.16 mm (both P>0.05).Conclusion
In this work, influences of gated/non-gated sequences, VENC and in-plane resolution on hemodynamic characteristics of blood flow in rat PC-MRI were investigated. Previous human study has showed the flow measurement is independent of whether the gated instrument is used or not. In animal studies, however, the usage of gated instrument can provide more reproducible results. VENC has insignificant influences on flow, WSS and artery area. To compromise the trade-off between accuracy and time-consuming, the resolution of 0.21 mm is suggested for extracting hemodynamic information about rat CCA.Acknowledgements
This work was supported by MOST-105-2815-C-039-033-B and MOST-105-2314-B-039-044-MY2.References
1. Peng SL, Wang FN, Yang TC, Hsu JC, Wu YC, Peng HH. Phase-contrast magnetic resonance imaging for the evaluation of wall shear stress in the common carotid artery of a spontaneously hypertensive rat model at 7T: Location-specific change, regional distribution along the vascular circumference, and reproducibility analysis. Magnetic resonance imaging. 2016;34:624-631 2.
2. Xu F, Ge Y, Lu H. Noninvasive quantification of whole-brain cerebral metabolic rate of oxygen (cmro2) by mri. Magnetic resonance in medicine. 2009;62:141-148 3.
3. Liu P, Xu F, Lu H. Test-retest reproducibility of a rapid method to measure brain oxygen metabolism. Magnetic resonance in medicine. 2013;69:675-681 4.
4. Peng SL, Ravi H, Sheng M, Thomas BP, Lu H. Searching for a truly "iso-metabolic" gas challenge in physiological mri. Journal of cerebral blood flow and metabolism : official journal of the International Society of Cerebral Blood Flow and Metabolism. 2016 5.
5. Lotz J, Meier C, Leppert A, Galanski M. Cardiovascular flow measurement with phase-contrast mr imaging: Basic facts and implementation. Radiographics : a review publication of the Radiological Society of North America, Inc. 2002;22:651-671
Figures