4880
4D flow MRI measurements in ex vivo beating pig hearts as a testing platform for transcathether aortic valve implantation1Department of Radiology, Academic Medical Center, Amsterdam, Netherlands, 2LifeTec Group, Eindhoven, the Netherlands, Netherlands, 3Department of Biomedical Engineering & Physics, Academic Medical Center, Amsterdam, Netherlands, 4Department of Cardiothoracic Surgery, Leiden University Medical Center, Leiden, Netherlands
Synopsis
In this 4D flow MRI study, ex vivo beating pig heart models in an MR-compatible setup mimicking human physiological conditions were used to investigate flow alterations after implantation of artificial valves designed for transcathether aortic valve implantations (TAVI). Two pig hearts had implanted TAVI valves (one with and one without attachment to the aortic sinuses) and were compared to five pig hearts without valve implantation. For both pig hearts with implanted valves, substantial aortic backflow, and thus paravalvular leakage, was observed. The ex vivo set-up presented is thus suitable for cardiac flow experiments.
Introduction
In elderly aortic stenosis patients transcathether aortic valve implantation (TAVI) has been developed as an alternative for surgery1. There is a wide variety in implantable TAVI valves available but the impact on cardiac hemodynamics through potential paravalvular leakage is unknown. In this study we propose to use 4D flow MRI to assess the hemodynamic performance of TAVI valves in ex vivo pig heart models under human physiological conditions.Methods
All hearts used in this feasibility study (n=7) were obtained from pigs that were slaughtered for human consumption. The protocols at the slaughterhouse and laboratory were in agreement with EC regulations 1774/2002 regarding the use of slaughterhouse animal material for research, supervised by the Dutch Government and approved by the associated legal authorities of animal welfare (Food and Consumer Product Safety Authority). The pig hearts were connected to the PhysioHeart platform2 (LifeTec Group BV, Eindhoven, The Netherlands), consisting of a coronary perfusion loop and a systemic circulation loop. For preparation, the pericardial sack was discarded and the azygotic vein and the inferior caval vein were ligated. A cannula with an internal diameter of 17mm was inserted into the aorta and fixed distal to the aortic valve. The pulmonary veins were removed to the level of the left atrium and ligated. A 27mm cannula was inserted into the left atrium and secured with a purse string suture. Similarly, a 19mm cannula was inserted into the pulmonary artery while the inferior and superior vena cava were removed and ligated, to close the right atrium2. After reinstating coronary perfusion and after defibrillation, the heart started contracting to reach sinus rhythm. Physiological pre- and afterload pressures mimicking human conditions were subsequently applied to the left ventricle (cardiac output: 5 liters pig blood per minute, systolic/diastolic pressure: 120/80mm Hg). The right ventricle was subjected to volume loading, resulting from return of coronary venous blood3. In one heart a 29mm CoreValve (Medtronic Inc., Minneapolis, Minnesota) was sewed in the aortic sinuses. In another heart a 26mm Edwards Sapien (Edwards Lifesciences LLC, Irvine, California) was implanted without attachment. Five hearts were scanned with native valves. A retrospectively cardiac gated 4D flow MRI examination on a 3T scanner (Ingenia, Philips Healthcare, Best, Netherlands) was performed: FOV=150x150x150mm3; spatial resolution=2.3x2.3x2.3mm3; TE/TR/FA: 2.2ms/5.2ms/8°; VENC=100cm/s; timeframes=24; k-t PCA4 R=3. Scan time was approximately 12 minutes. Velocity pathlines were visualized in GTFlow (Gyrotools, Zurich, Switzerland) by thresholding the images for high magnitude, and high and low velocity values. Stroke volume of in- and outflow was quantified by drawing regions of interest in the aorta, pulmonary artery and left atrial inflow.Results
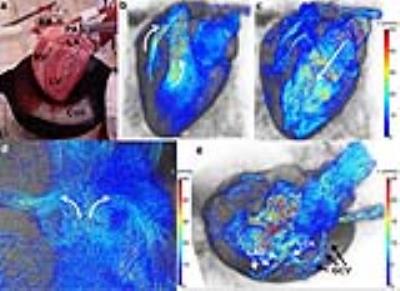
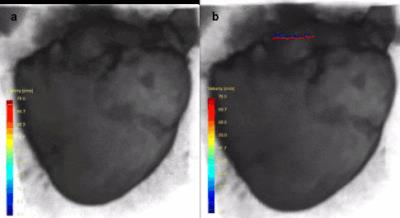
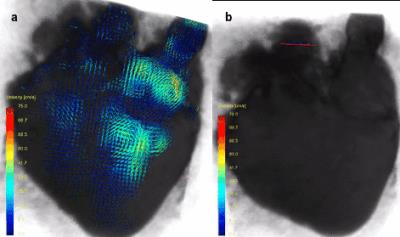
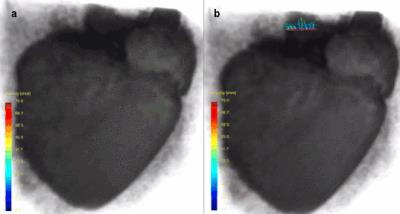
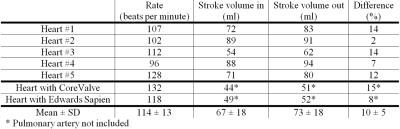
In figure 1, an ex vivo pig heart without valve implantation is displayed, with characteristic velocity pathline visualizations in the ejection and filling phase. Furthermore, vortical flow in the aortic sinuses and coronary flow can be observed. In figure 2a, the same heart is displayed with time-resolved velocity pathlines over the cardiac cycle. In figure 2b, the aortic outflow is displayed. No backflow is observed. In figure 3a, flow in the pig heart with the implanted CoreValve is shown. Susceptibility artefacts from the stent in the valve obscure flow in the aorta. However, more distal from the valve, the outflow can be visualized (figure 3b). Some back flow into the aorta can be seen. In figure 4a, flow in the pig heart with the implanted Edwards Sapien valve is displayed, with a close-up of aortic flow in figure 4b. Again, some of the flow in the aorta is obscured by susceptibility artefacts from the stent. However, in figure 4b a large amount of backflow into the heart can be seen. In table 1, the stroke volumes in and out the heart and the difference are given. Stroke volumes were lower for the heart with implanted valves. The mean error between in and outflow volumes was 10%.Discussion
In both pig hearts with implanted artificial valves, paravalvular leakage in the form of aortic backflow was seen. However, in patients with stenotic valves, these valves are kept in place by calcifications in and around the valve. In the set-up presented here, hearts of young pigs were used, and no calcifications were present. Thus, artificial valves tend to migrate during heart beats. Especially in the case for the Edwards Sapien, this may have led to large amounts of paravalvular leakage.Conclusion
In this study, the first MR-compatible set-up for 4D flow MRI measurements in ex vivo beating pig hearts loaded to human physiological conditions for testing hemodynamic performance of artificial valves for TAVI procedures was demonstrated.Acknowledgements
STW Grant CARISMA 11630: PAPAVERReferences
1. Nishimura RA, Otto CM, Bonow RO, et al., AHA/ACC Guideline for the Management of Patients With Valvular Heart Disease: Executive Summary: A Report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines. J Am Coll Cardiol, 2014
2. Hart J de, Weger A de, Tuijl S van, Stijnen JMA, Broek CN van den, Rutten MCM, Mol BA de. An ex vivo platform to simulate cardiac physiology: a new dimension for therapy development and assessment. Int J Artif Organs 2011, 34:495–505
3. Schampaert S, ’t Veer M van, Rutten MCM, Tuijl S van, Hart J de, Vosse FN van de, Pijls NHJ. Autoregulation of coronary blood flow in the isolated beating pig heart. Artif Organs 2013;37:724–30
4. Pedersen H, Kozerke S, Ringgaard S, Nehrke K, Kim WY. k-t PCA: Temporally constrained k-t BLAST reconstruction using principal component analysis. Magn Reson Med 2009;62:706–716.
Figures