4875
MRI/PET of Myocardial Extracellular Volume in a Canine model of Chemotherapy1Radiology and Imaging Sciences, National Institutes of Health, Bethesda, MD, United States, 2Division of Veterinary Resources, National Institutes of Health, 3Department of Radiology, Johns Hopkins Hospital, MD, United States
Synopsis
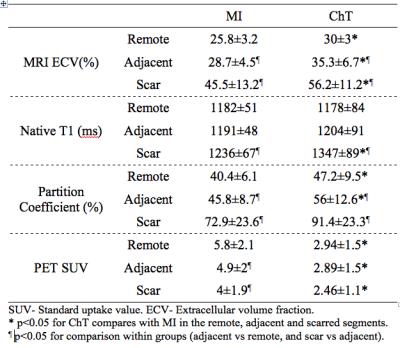
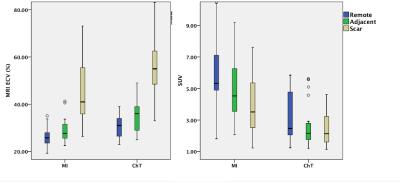
We used simultaneous MR/FDG PET to measure the myocardial extracellular volume (ECV) and glucose metabolism (estimated by standard uptake value, SUV) in a canine model of chemotherapy (ChT); comparison was made to ECV in relationship to a myocardial infarct (MI) models. MRI ECV in the ChT group was elevated by 16% and 23% compared to the MI group in the remote and adjacent myocardial segments, respectively. PET SUV in the ChT group was reduced by 49% and 41% compared to the MI group in the remote and adjacent myocardial segments, respectively. Difference was also observed in the MRI partition coefficient but not in the native T1.
Introduction
Alterations in the tissue composition of the myocardium are related to adverse myocardial function. The most common insult to the myocardium is myocardial infarction (MI); MI is associated with LV remodeling and alterations of the extracellular matrix even in regions remote from the myocardial infarction territory. Chemotherapy (ChT) can induce acute (hypotension, arrhythmias) and chronic (heart failure) cardiotoxicity. Recent studies suggest that cancer survivors frequently have cardiac dysfunction that might be assessed with MRI (1-2). However, little is known about the precise mechanism of ChT induced myocardial dysfunction, partially because of the lack of an adequate large animal model of ChT-induced cardiomyopathy. The goal of this study was to develop a novel canine model of chemotherapy-induced cardiomyopathy and to use high sensitivity imaging modalities (MRI/PET) to identify early adverse changes in myocardial structure and function. Specifically, we compare changes in the myocardial interstitium and glucose metabolism in the areas that were directly or indirectly affected by the applied intervention. Comparison was made to animals with chronic myocardial infarction.Methods
Adult male mongrel dogs (weighted 26-30 kg) were used in the MI model and ChT model. In the chemotherapy model, weekly infusions of 7.5-15mg of doxorubicin were delivered for 4 weeks via a chronic indwelling intracoronary catheter. Imaging was performed 8-12 weeks after the first infusion. For comparison, a standard occlusion/ reperfusion model of MI was induced by vascular clamp occlusion of the left anterior descending (LAD) artery for 90 minutes followed by reperfusion. Imaging was performed 6-8 weeks after the surgical procedure. Dogs were anesthetized, intubated and ventilated in preparation for the imaging studies. Cardiac images including left ventricular (LV) function, late gadolinium enhancement (LGE), and T1 mapping were acquired on a 3T integrated PET/MR scanner. PET images were simultaneously acquired after injecting 5 mCi of FDG 83±30 minutes before, to monitor myocardial metabolism. PET images were reconstructed and registered with MRI T1 maps. Based on the AHA 16 segment model, MRI myocardial extracellular volume (ECV) maps (reconstructed using pre and post contrast T1 maps, and hematocrit obtained during MRI) and PET images were categorized into scar (with LGE), adjacent (no LGE, proximal to scar segments), and remote (no LGE, distal to scar) segments. Segmental MRI ECV, partition coefficient, native T1, and PET standard uptake value (SUV) were recorded for comparison between MI and ChT groups and tissue compositions using generalized estimation equations (GEE). Significance was declared as p<0.05.Results
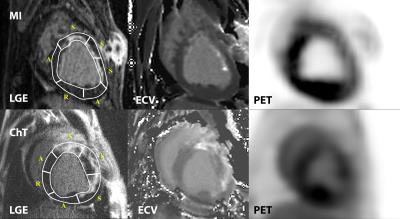
Six ChT dogs survived ChT during the protocol (75% survival) and were included in the final analysis. Six ChT dogs survived ChT during the protocol (75% survival) and were included in the final analysis. Ten animals had successful induction of MRI with LGE > 5%. Table 1 summarizes the animals’ characteristics stratified model. ChT dogs had significantly larger LV volumes (40% EDV increase). A focal MRI LGE injury from ChT was present in all ChT animals. Figure 1 demonstrates representative LGE images, ECV maps, and PET images from each group. In presumptively normal tissue remote from the vascular territory of the catheter infusion, MRI ECV in the ChT group was elevated by 16% (remote) and 23% (adjacent) compared to the MI group. PET SUV in the ChT group was reduced by 49% and 41% compared to the MI group in the remote and adjacent myocardial segments, respectively. Differences between groups were also observed in the partition coefficient but not in the native T1. MRI ECV in the adjacent segments was significantly higher than that in the remote segments in both MI and ChT groups. In the MI dogs, PET SUV was decreased by 16% in the adjacent segments compared to the remote area. In contrast, there was no difference in SUV between remote and adjacent tissue in ChT canines. Comparison is shown in Table 2 and Figure 2.Conclusions
Doxorubicin-induced cardiomyopathy by intracoronary infusion in myocardium results in expansion of the extracellular volume and reduction of glucose metabolism in infarcted, adjacent, and remote myocardium in the subacute setting. These changes were diffuse and more prominent than corresponding changes related to subendocardial myocardial infarction. Furthermore, MRI ECV showed a gradient transition from scarred to remote myocardium, which was an indication of systematic tissue change from focal replacement fibrosis to diffuse fibrosis. The intracoronary infusion model of chemotherapy may represent a relatively rapid approach (8-12 weeks) to develop a non-human model of nonischemic cardiomyopathy.Acknowledgements
The authors thank Mr. Robert Evers in the assistance of MR/PET scanning, and all Veterinary Resources staff for their valuable contributions.References
1. Jordan JH, Vasu S, Morgan TM, D'Agostino RB, Jr., Melendez GC, Hamilton CA, Arai AE, Liu S, Liu CY, Lima JA, Bluemke DA, Burke GL and Hundley WG. Anthracycline-Associated T1 Mapping Characteristics Are Elevated Independent of the Presence of Cardiovascular Comorbidities in Cancer Survivors. Circ Cardiovasc Imaging. 2016;9. 17.
2. Tham EB, Haykowsky MJ, Chow K, Spavor M, Kaneko S, Khoo NS, Pagano JJ, Mackie AS and Thompson RB. Diffuse myocardial fibrosis by T1-mapping in children with subclinical anthracycline cardiotoxicity: relationship to exercise capacity, cumulative dose and remodeling. J Cardiovasc Magn Reson. 2013;15:48.
Figures