4873
Type 1 Diabetic Hearts show Unexpected Biphasic Metabolic and Functional Progression as Evaluated with Hyperpolarised [1-13C]Pyruvate and CINE MR.1Department of Physiology, Anatomy & Genetics, University of Oxford, Oxford, United Kingdom
Synopsis
Type 1 diabetes patients are insulin deficient resulting in hyperglycaemia. Diabetic patients have a higher risk of cardiovascular diseases. In this study the progression of cardiac metabolic and functional decline was followed in a streptozotocin (STZ) induced Type 1 diabetic model. Flux through pyruvate dehydrogenase was significantly decreased at 2 and 6 weeks post STZ injection. Interestingly, the incorporation of the 13C-label from pyruvate into lactate and alanine was decreased at 2 weeks, but significantly increased at 6 weeks. Cardiac output was normalized after 6 weeks. Such studies will allow a better understanding of the interactions between metabolism and function in the diabetic heart.
Introduction
Type 1 diabetes mellitus patients are insulin deficient resulting in hyperglycaemia. Diabetes is known to be associated with a high risk of cardiovascular diseases [1]. Cardiac disease is associated with changes in metabolism, and understanding the balance between different energy sources, can be core to understanding the pathophysiology of cardiac diseases. The purpose of this study was to follow the progression of cardiac metabolic and functional decline in a rodent model of Type 1 diabetes induced with a high dose injection of the β-cell toxin, streptozotocin (STZ).Methods
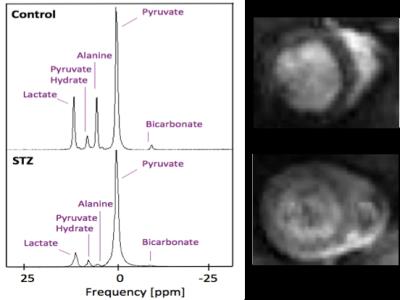
8 healthy Male Wistar rats (~230 g) were fasted overnight and randomly divided in 2 groups; (1) Diabetic rats injected with STZ (55 mg/Kg) and (2) Control rats injected with citrate buffer. At 2 and 6 weeks after the STZ injection animals were anesthetized with isofluorane and hyperpolarized 13C MRS and CINE MR imaging were performed for metabolic and functional assessment of the heart [2,3]. 13C MR pulse-acquired cardiac spectra were acquired over 60s after injection of hyperpolarised [1-13C]pyruvate (repetition time 1s; excitation flip angle 15°; sweep width 13,021 Hz; acquired points 2,048; frequency centered on the C1 pyruvate resonance) and spectra were summed over 30s (Fig. 1) from the first appearance of pyruvate and analysed with JMRUI [2]. Eight to ten short-axis CINE slices (slice thickness: 1.6 mm, matrix size = 128 × 128, TE/TR = 1.67/4.6 ms, flip angle 15°, number of averages: 4) were acquired (Fig. 1) with a FLASH sequence for the assessment of cardiac function and analysed with ImageJ.Results
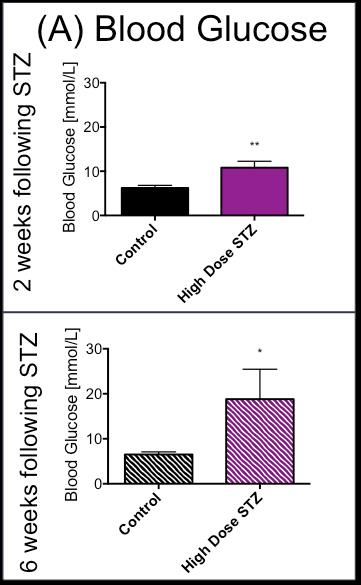
In line with the development of Type 1 diabetes, blood glucose was significantly and progressively increased at 2 and 6 weeks after the STZ injection (Fig. 2). With the development of diabetes, flux through pyruvate dehydrogenase, as indicated by reduced conversion of hyperpolarized pyruvate into bicarbonate, was significantly decreased at both time-points studied (Fig. 3). Correlation between blood glucose measurements and bicarbonate signal were observed at 2 weeks (R2=0.82, p = 0.002) and 6 weeks (R2 = 0.42, p = 0.06) (Fig. 4) Interestingly, the incorporation of the 13C label from pyruvate into both lactate and alanine was significantly decreased at 2 weeks, but significantly increased at 6 weeks after the STZ injection (Fig. 3). From a functional perspective, stroke volume and cardiac output were both decreased in the diabetic heart after 2 weeks, but this functional depression was reversed 6 weeks after the onset of diabetes (Fig. 5).Discussion and Conclusion
This study suggests a progressive change in cardiac metabolism and function following the onset of Type 1 diabetes. Initial depression of cardiac function occurs in combination with suppression in the metabolic conversion of pyruvate to both lactate and alanine. In contrast the restoration of normal cardiac function at 6 weeks after the onset of diabetes coincides with an elevation in the conversion of hyperpolarized pyruvate into lactate and alanine. This may suggest that some functional benefit is obtained from the ability to redirect pyruvate into alternative metabolic pathways. This study has shown the potential for hyperpolarized MRS and CINE MRI to study the progressive changes in cardiac metabolism and function that occur alongside the development of Type 1 diabetes. Such studies will allow a better understanding of the interactions between metabolism and function in the diabetic heart and may provide new insight into novel therapeutics.Acknowledgements
No acknowledgement found.References
[1] T. Doenst et al. Circ. Res., vol. 113, no. 6, pp. 709–24, Aug. 2013
[2] Dodd et al. Cardio. Res., vol. 95, pp. 69-76, May. 2012
[3] Tyler et al. Magn. Res. in Med., vol. 60, pp.582-587, Apr. 2008
[4] Vanhamme L et al. J. Magn. Reson, 129, pp. 35-43, 1997.
Figures
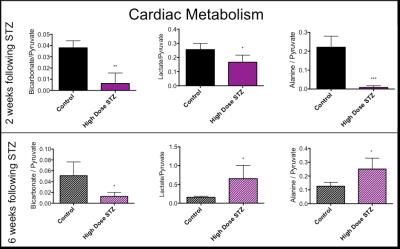
In vivo effects of infusion of hyperpolarized [1-13C]pyruvate into control and STZ injected rats. Pyruvate’s metabolic products (bicarbonate, lactate and alanine) were summed over 60 seconds and normalised to the pyruvate peak. Unpaired t-test *p < 0.05, **p < 0.01, ***p < 0.001.
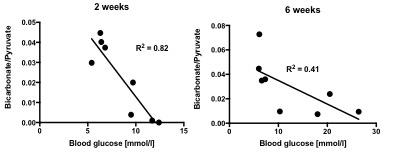
Correlation between blood glucose levels measured in fasted state and bicarbonate/pyruvate ratio signal summed over 30s of spectra acquisition at 2 weeks post STZ injection (left) and 6 weeks post STZ injection (right).
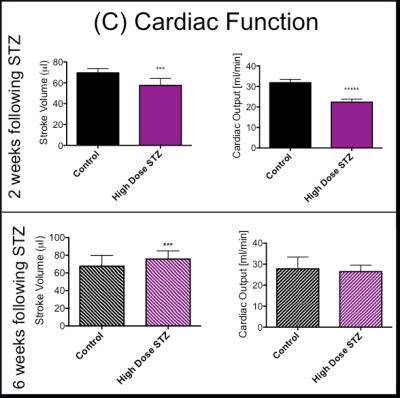
Cardiac Function obtained from CINE MR imaging, stroke volume and cardiac output were assessed in Control rats and STZ rats. Unpaired t-test *p < 0.05, **p < 0.01, ***p < 0.001.