4872
Reversible Changes in Cardiac Function and Metabolism in an Inducible Mouse Model of Type 1 Diabetes1University of Oxford, Oxford, United Kingdom
Synopsis
Heart disease is the leading cause of death in Type 1 diabetic patients, however the mechanistic link has not been fully established. In this study an inducible and reversible mouse model of Type 1 diabetes was used. Depression of cardiac function and the increase in blood glucose occur in combination with suppression of pyruvate to bicarbonate conversion. By reversing diabetes with Glibenclamide, cardiac function and blood glucose concentration was restored. This study demonstrated changes that occur alongside the development of a reversible model of Type 1 diabetes and how the action of Glibenclamide can affect metabolism and function of the heart.
Introduction
Heart disease is the leading cause of death in Type 1 diabetic patients, however the mechanistic link has not yet been fully established [1]. In this study an inducible and reversible mouse model of Type 1 diabetes was used. The mouse model has a tamoxifen inducible mutation in a subunit of the pancreatic β-cell KATP channel that results in continuous opening of the channel, thereby preventing insulin release and leading to hyperglycaemia. Subsequent treatment with the sulphonylurea; Glibenclamide, can reverse hyperglycaemia in the mouse model by closing the KATP channel [2]. The purpose of this study was to follow mice at baseline, through the development of diabetes and subsequently through treatment with Glibenclamide to investigate the effects on cardiac metabolism and function in a reversible model of Type 1 diabetes.Methods
Ten healthy mice were imaged at 3 time points, baseline, 2 weeks after the induction of diabetes, and 2 weeks following treatment with Glibenclamide (2.5mg). The animals were imaged for both metabolic and functional assessment of the heart [3,4]. The mice were imaged with hyperpolarized [1-13C]pyruvate MRS performed on a 7T horizontal bore MRI system. 13C MR pulse-acquire cardiac spectra (repetition time: 1s, flip angle: 15°, sweep width 8,012Hz; acquired points 2,048; frequency centred on the C1 pyruvate resonance) were acquired over 60s after injection of hyperpolarized pyruvate (30 min of hyperpolarization at ~1K). All spectra were analysed with JMRUI [5]. Spectra were summed over 30s from the first appearance of the pyruvate peak. CINE imaging was performed on an 11.7T vertical bore MRI system. Seven-eight short-axis CINE slices (slice thickness: 1mm, field of view: 2.56 x 2.56 cm, number of averages: 4) were acquired (Fig. 2) with a FLASH sequence and analysed with ImageJ.Results
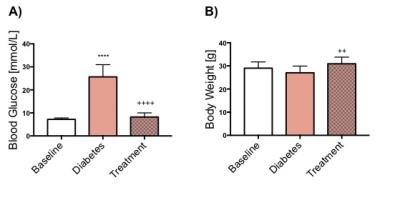
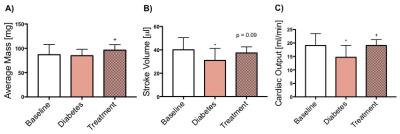
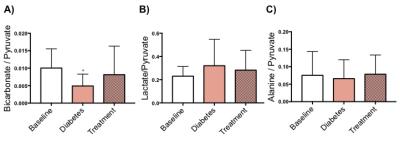
Blood glucose levels were significantly increased with the development of diabetes, however, the blood glucose levels were restored to normal following treatment with Glibenclamide (Fig. 1A). Body weight did not change with development of diabetes but the animals gained weight significantly following treatment (p=0.006) (Fig. 1B). Cardiac function decreased with the development of diabetes as shown by a significant decrease in both stroke volume (SV) and cardiac output (CO). Cardiac function, measured by CO (p=0.01) and SV (p=0.09), was restored following treatment with Glibenclamide (Fig. 3). Average heart mass was unchanged by diabetes but increased significantly with treatment (p=0.04) (Fig. 3). With the development of diabetes, flux through pyruvate dehydrogenase was significantly decreased in diabetes (p=0.03), as indicated by reduced conversion of hyperpolarized pyruvate into bicarbonate. However, the observed decrease in bicarbonate signal in diabetes was restored to baseline levels following treatment. The incorporation of the 13C label from pyruvate into both lactate and alanine was unchanged at all time points.
Discussion and Conclusion
Depression of cardiac function, as seen by decreased SV, CO, and the increase in blood glucose concentration occur in combination with a significant suppression in the metabolic conversion of pyruvate to bicarbonate. By reversing diabetes with Glibenclamide, cardiac function and blood glucose concentration was restored. This study has demonstrated that hyperpolarized MRS and CINE MRI can be used successfully to study changes in cardiac metabolism and function that occur alongside the development of a reversible model of Type 1 diabetes and how the reversible action of sulphonylureas can affect metabolism and function of the heart. Further studies using these techniques will allow a better understanding of the interactions between metabolism and function in the diabetic heart and may provide new insight into novel therapeutics to heart disease in diabetic subjects.Acknowledgements
No acknowledgement found.References
[1] T. Doenst et al. Circ. Res., vol. 113, no. 6, pp. 709–24, Aug. 2013
[2]FM. Ashcroft et al. Diabetologia, vol. 55, no. 4, pp 1195-1204, Apr. 2012
[3] Dodd et al. Cardio. Res., vol. 95, pp. 69-76, May. 2012
[4] Tyler et al. Magn. Res. in Med., vol. 60, pp.582-587, Apr. 2008
[5] Vanhamme L et al. J. Magn. Reson, 129, pp. 35-43, 1997.
Figures