4870
Multi-parametric MRI of Murine Unilateral Ureter Obstruction1Institute of Imaging Science, Vanderbilt University, Nashville, TN, United States, 2Radiology and Radiological Sciences, Vanderbilt University, Nashville, TN, United States, 3Division of Nephrology and Hypertension, Vanderbilt University, Nashville, TN, United States
Synopsis
Multi-parametric MRI techniques may allow the assessment of renal injury and function in a sensitive and objective manner. This study aimed to evaluate an array of MRI methods that exploit endogenous contrasts for their sensitivity in detecting abnormal features associated with kidney disease in a murine model of unilateral ureter obstruction (UUO). Diffusion weighted imaging (DWI), quantitative magnetization transfer (qMT), chemical exchange saturation transfer (CEST), and nuclear Overhauser enhancement (NOE) provide specific information about the cellular and molecular changes produced by UUO.
Purpose
Quantitative, multi-parametric MRI techniques may allow the assessment of renal injury and function in a sensitive and objective manner. This study aimed to evaluate an array of MRI methods that exploit endogenous contrasts including quantitative magnetization transfer (qMT), chemical exchange saturation transfer (CEST), nuclear Overhauser enhancement (NOE) and diffusion weighted imaging (DWI), for their sensitivity and specificity in detecting abnormal features associated with kidney disease in a murine model of unilateral ureter obstruction (UUO).Methods
MRI scans were performed in anesthetized wild type (WT) mice 3 or 6 days after UUO (N=8) at 7T. All procedures were approved by the Institutional Animal Care and Use Committee of Vanderbilt University. Anesthesia was induced and maintained with a 1.5%/98.5% isoflurane/oxygen mixture, and a constant body temperature of 37.5 ºC was maintained using heated air flow. Quantitative MRI data were obtained from a single 1-mm axial slice incorporating both kidneys. QMT data were collected using a 2D MT-weighted spoiled gradient recalled-echo sequence (TR 24 ms, flip angle = 7°, resolution = 0.167x0.167x1 mm3, 16 acquisitions). Gaussian-shaped saturation pulses (flip angles = 220° and 820°, pulse width = 10 ms) were used, with 7 different RF offsets between 1 and 80 kHz with a constant logarithmic interval. CEST acquisitions were obtained using a continuous wave (CW) CEST sequence with a 5.0 s irradiation pulse followed by 2-shot spin-echo echo-planar-imaging (SE-EPI, TR = 7.5 s, TE = 17.6 ms, resolution = 0.5x0.5x1 mm3). Z-spectra were acquired with RF offsets from -1500 Hz to 1500 Hz with an interval of 50 Hz and saturation pulse amplitude BCW = 1.0 μT. Reference scans were performed at the beginning and the end of the acquisitions with RF offset = 100 kHz. DW imaging was performed by using a SE-EPI sequence (TR/TE=3000/38 ms, resolution 0.25x0.25x1mm3), and diffusion gradients (duration/separation = 5/20 ms) were on three axes with 11 b-values ranging from 0 to 1000 sec/mm2. In addition, DWI scans were collected twice, each with opposite gradient polarity, and averaged to eliminate the presence of gradient cross-terms that may influence ADC measurements. Fat saturation was applied at RF offset -1042 Hz. After MRI and euthanasia, paraffin tissue sections were stained with Masson trichrome using standard procedures. All imaging data were analyzed using MATLAB. The model of Ramani et al.1 was applied to derive qMT parameters. CEST Z-spectra were fit to a 6-pool model consisting of Lorentzian peaks to estimate the magnitudes of CEST effects at 1.2, 2.2 and 3.5 ppm RF offsets, direct saturation (DS) effects at 0.0 ppm RF offset, and nuclear Overhauser enhancement (NOE) effects at -1.6 and -3.5 ppm RF offsets.2 Apparent diffusion coefficient (ADC) maps were obtained from mono-exponential fitting to the data using b-values > 300 sec/mm2 to avoid intra-voxel incoherent motion (IVIM) effects in kidneys.Results
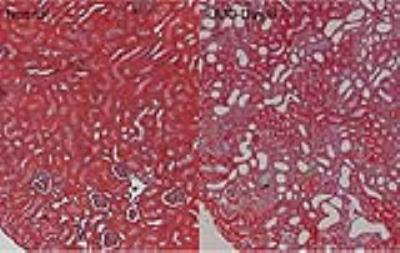
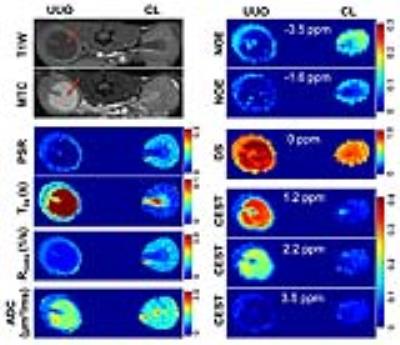
UUO progression was accompanied by tubular cell death, tubular dilation, urine retention, and interstitial fibrosis in renal cortex (Fig. 1). The obstructed urine showed very low pool size ratio (PSR), low longitudinal relaxation rate (R1obs), low NOE effects around -1.6 and -3.5 ppm RF offsets, very long transverse relaxation times for free water pool (T2a), high DS effect, large CEST effects for fast exchanging proton pools around 1.2 and 2.2 ppm RF offsets, low CEST effects for amide proton pools around 3.5 ppm RF offset, and high ADC (Fig. 2). All the above measured MRI parameters in the contralateral (CL) cortical region remained unaltered compared to normal kidneys. Compared to the CL kidney, the UUO kidney showed increase of T2a, and decrease of R1obs, PSR, and NOE in the cortical region. While the DS increased in the cortex of UUO kidney, ADC of UUO kidney decreased (Fig. 2). No significant changes in CEST effects were observed for the cortical region of UUO kidney (Fig. 2). Strong regional correlations were observed between MRI parameters, for both UUO and CL kidney.Discussion
In the cortical region, decreased PSR, NOE, R2a and R1obs values could be related to tubular cell death, tubular dilation, and urine retention in the cortex of UUO kidneys, while decreased ADC could be associated with urine obstruction, tubular atrophy and fibrosis in renal cortex. Increased DS is associated with the tubular dilation and urine retention.Conclusion
Measurements of multiple MRI parameters provide specific information about the molecular and cellular changes produced by UUO. These observations together provide an endogenous, non-invasive and quantitative evaluation of renal injury and function during UUO progression.Acknowledgements
We thank Mr. Fuxue Xin, Mr. Ken Wilkens, Dr. Daniel C. Colvin, Mr. Jarrod True, and Dr. Mark D. Does in the Center for Small Animal Imaging at Vanderbilt University Institute of Imaging Science for assistance.References
1. Ramani, A., et al., Precise estimate of fundamental in-vivo MT parameters in human brain in clinically feasible times. Magn Reson Imaging, 2002. 20(10): 721-31.
2. Wang, F., et al., Mapping murine diabetic kidney disease using chemical exchange saturation transfer MRI. Magnetic resonance in medicine, 2016. 75(4): 1685-1695.
Figures