4869
Self resonated clip for in-utero mouse embryonic MRI1Radiology, NYU - School of Medicine, New York, NY, United States
Synopsis
In this study, we introduce a newly designed technique called clipping to help stabilize the imaged embryos. Furthermore, this setup has potential for high-throughput imaging of live embryos using large volume coils in combination with individual inductive coupling loops for each embryo. Our results showed that the clipping technique secure the embryo for an extended imaging time of more than 90 minutes. The combination of volume coil and inductive coupling loop [ref] helps increasing the signal to noise ratio (SNR) for more than 3 folds compare to the volume coil alone and closely reach the level of commercial 4 channel received only surface coil.
Introduction
In utero mouse imaging of living embryo can be very useful for the noninvasive characterization of various background strains during embryogenesis, for phenotyping transgenic models, or for studying developmental diseases [1]. In this case, imaging embryos is inherently prone to global periodic abdominal movements of the mother associated with respiratory breathing, cardiac contractions and bowel movement. In addition to the injection of preterm labor drug to minimize uterine contractions, the predictable direction of displacement of the abdomen can be minimized by orienting the phase encoding orthogonal to the direction of motion. Furthermore, the cyclical nature of these movements can be accounted for through either image gated-acquisition [2] or through the use of self-gated navigator sequence strategies [3]. However, embryos also experience spontaneous movements within the uterus that are unpredictable and that can be minimized by surgically securing individual subject [2] or through serial coregistration of rapidly acquired three-dimensional images [4,5]. In the latter, the acquisition can be very demanding for the gradient coil performance and also require off-line complex image registration. In this study, we introduce a newly designed and 3D printed clip-based setup to be used noninvasively in utero. To this effect, a set of clips of varying size were tailored to snuggle around individual embryos at various stages of the development in order to help stabilize their spontaneous movements. We demonstrate that this simple strategy can be effective in imaging individual embryo when combined with respiratory gating acquisition that were both tested successfully using whole body and surface coils. Importantly, we also used this clip as a support to further integrate a self-resonant loop that is effectively circumventing the secured embryo. This clip-mounted self-resonant coil is then inductively coupled to a much larger volume resonator [6-9] that can accommodate large pregnant mice. This latter strategy helps increase the signal to noise ratio (SNR) by 3 folds compared to the volume coil alone and almost match the sensitivity of a commercial 4 channel receive-only surface coil coupled to transmit-only coil closely fitting the bore of the magnet.Materials and methods
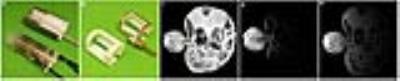
A set of clips were designed and 3D printed withsize and thickness were scaled appropriately for each stage of the embryos. In addition, a self-resonant coil was integrated as part of the clip using copper tape looped to a non-magnetic variable capacitor (Voltronics) and tuned to a frequency slightly lower but within re-adjustable range (in this case 296Mhz ) to the Larmor frequency (300MHz in the current 7-Tesla scanner) [6-9]. Animals: Mice were maintained according to IACUC approved protocols at the NYU School of Medicine. To illustrate the effectiveness of our clip-based strategy in reducing spontaneous motion of individual embryos, pregnant mice were administered intra-peritoneally (i.p.) with 30mM MnCl2 in isotonic saline (0.9% NaCl in water) [2, 10] using dose of 0.16 mmol/kg (33 mg/kg) per body weight. Manganese-enhanced MRI was used to generate highly detailed embryonic CNS structures as previously shown [2,10]. For the comparison of the RF coil performance, we tested a Bruker 4-channel phased array receive-only surface coil (T11483V3 / 99, 30x30mm) (figure 2a top) to a whole mouse body dual Litz cage (L: 76mm, ID: 38mm) deisgned in house. sequence for MEMRI embryos we used a T1-weighted 3D FLASH: 125um isotropic : TE/TR 3.5/30ms, NA 3, matrix 160x240x160, FOV: 20x30x20mm, FA: 30o, BW 75kHzg. For the Performance comparison: 3D FLASH 267um isotropic: TE/TR 4.75/30ms, NA 3, matrix 150x322x150, FOV: 40x86x40mm, FA: 30o, BW 75kHzResults and discussions
The high resolution 3D MEMRI scan (figure 1d) showed that the clip helps to secure the imaged embryos in position during the whole scanning time. In order to increase the throughput, the clipping setup was tested in the homemade whole body Litzcage coil. Due to the large size of the coil, hence the lack of filling factor, a drop of more 3.6 folds in SNR was observed. This drop in signal can be compensated by introducing an inductive coupling loop wrap closely to the region of interest (the imaged embryo). This inductive coupling loop helped to focus the magnetic energy to a more confined volume [4-8] (increasing the filling factor) (figure 2d, SNR of 608) that lead to a SNR ~3.2 fold higher compare to the whole body coil alone (figure 2c, SNR of 190) and only 12% less than the commercial surface coil (figure 2e, SNR of 688).Conclusions
Our design demonstrated a good performance in securing the embryo for in utero MRI. The design can be integrated into any MRI system using existing commercial hardware.Acknowledgements
This work was performed at the Preclinical imaging core; a shared resource partially supported by the NYUCI Center Support Grant, “NIH/NCI 5P30CA016087”, the NIBIB Biomedical Technology Resource Center (NIH P41 EB017183) and by NIH grant UL1 TR00038 from the National Center for Advancing Translational Sciences (NCATS).References
1. Nieman BJ. 3D. Curr Opin Genetics Dev. 2011; 21(5):638–646.
2. Deans AE. Magn Reson Med. 2008 Jun;59(6):1320-8. doi: 10.1002/mrm.21609.
3. Nieman BJ. Magn Reson Med. 2009 May;61(5):1148-57. doi: 10.1002/mrm.21945.
4. Berrios-Otero CA. Magn Reson Med. 2012 Jan;67(1):251-7. doi: 10.1002/mrm.22991.
5. Parasoglou P. NMR Biomed. 2013 Feb;26(2):224-31. doi: 10.1002/nbm.2843. Epub 2012 Aug 22.
6. Bilgen M. Biomed. Eng. Online 2006; 5: 3.
7. Utz M. J. Magn. Reson. 2009; 198: 132–136.
8. Banson ML. Invest. Radiol. 1992; 27: 157–164.
9. Glover PM. Magn. Reson. Med. 1994; 31: 423–428.
10. Wadghiri YZ. NMR Biomed. 2004 Dec;17(8):613-9.
Figures