4864
Evaluation of compressed sensing for 3D T1-weighted fat-suppressed breast MRI1Department of Medical Physics, University of Wisconsin, Madison, WI, United States, 2Department of Radiology, University of Wisconsin, Madison, WI, United States, 3Global MR Applications and Workflow, GE Healthcare, Hino, Japan, 4Global MR Applications and Workflow, GE Healthcare, Waukesha, WI, United States, 5Carbone Cancer Center, University of Wisconsin, Madison, WI, United States, 6Global MR Applications and Workflow, GE Healthcare, Madison, WI, United States
Synopsis
Spatial resolution has typically been prioritized at the expense of temporal resolution in the setting of dynamic contrast-enhanced breast MRI. In this work, we evaluated the use of compressed sensing (CS) with intermittent fat suppression for improved temporal resolution by comparing quality of fat suppression and overall image quality between a sequence accelerated using CS to one without CS.
Introduction
Spatial and temporal resolution present competing demands in MRI. In clinical dynamic contrast-enhanced (DCE) breast MRI, spatial resolution is typically prioritized over temporal resolution due to the importance of assessing lesion morphology.1 However, recent advances in MRI may help improve the temporal resolution of DCE breast MRI while maintaining the same high spatial resolution. One such advancement, compressed sensing (CS), allows for the reconstruction of MR images from undersampled k-space data, thus improving the temporal resolution.2-4 However, this technique requires incoherent sampling, which can be particularly challenging when combined with intermittent fat saturation. In this work, we present a technique that uses compressed sensing in order to accelerate the acquisition while also enabling intermittent fat suppression.Methods
Nineteen patients undergoing a clinical breast MRI exam consented to this IRB-approved, HIPAA-compliant study. Patients were scanned on a 3.0 T scanner (Discovery MR 750w, GE Healthcare, Waukesha, WI) using a 16-channel breast coil (Sentinelle, Invivo, Gainesville, FL). Single phases of two 3D T1-weighted spoiled gradient echo based acquisitions, one with CS and one without CS (non-CS), were acquired at the end of the clinical breast MRI exam, approximately ten minutes after the injection of contrast agent. The order of the two research sequences was alternated to account for the difference in post-contrast timing.
To reduce scan time, data-driven parallel imaging was used to uniformly undersample outer k-space. For the CS scans, outer k-space was further undersampled pseudo-randomly using a Gaussian probability function, which is well-suited for compressed sensing reconstruction.5 A spectrally-selective adiabatic fat suppression pulse was used for both research sequences. However, in order to achieve acceptable fat suppression with CS, the timing of the intermittent fat suppression pulses was modified to account for the incoherent sampling required for CS. The spatial resolution of both scans was 0.7 x 0.7 x 1.4 mm3, while the temporal resolutions for the CS and non-CS scans were 85 and 109 seconds, respectively.
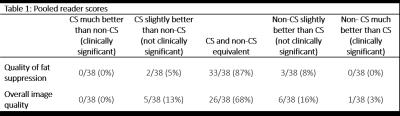
Two readers specializing in breast MRI performed a blinded review of images during two sessions, one to assess the quality of fat suppression and one to assess overall image quality. Images were scored as: 1) CS much better than non-CS (clinically significant), 2) CS slightly better than non-CS (not clinically significant), 3) CS and non-CS equivalent, 4) non-CS slightly better than CS (not clinically significant), and 5) non-CS much better than CS (clinically significant). During each session, images were reviewed side-by-side, and the position of the images on the screen (left or right) was randomized. During each session, the order of the patients was also randomized. Readers were blinded to the sequence type and results of the other reader.
Results
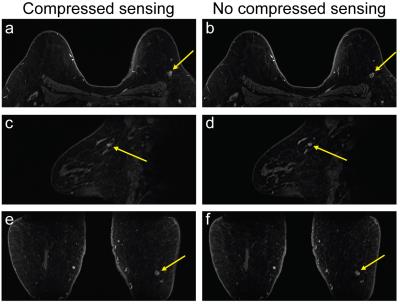
CS reduced the scan time by 24 seconds (~22%) for a single phase compared to the non-CS sequence. Most cases were scored as having equivalent fat suppression (16/19 and 17/19 for each reader). Of the cases that did not have equivalent fat suppression, the difference in fat suppression quality was never considered clinically significant (Table 1). Similarly, most cases were scored as having equivalent image quality (13/19 for both readers). One reader scored a single case as a clinically significant difference, with the non-CS images having better image quality than the CS images. While no obvious motion was present during the acquisition of the non-CS scans, evidence of patient motion was visible during the acquisition of the CS scans and likely contributed to the difference in image quality (Figure 1). All other differences in image quality were not clinically significant. A representative case is shown in Figure 2.Discussion and Conclusion
Combining the acquisition with CS undersampling and intermittent fat suppression provided a 22% improvement in temporal resolution while maintaining the quality of fat suppression and overall image quality compared to the sequence without CS. Although one case was considered to have a clinically significant difference in overall image quality, patient motion during the CS acquisition likely contributed to the decreased image quality. This study evaluated a single phase post-contrast; however, a full DCE exam would likely assist in identifying the presence of motion. Further work is needed to demonstrate the clinical utility of this technique as well as to evaluate this technique in patients with malignant lesions.Acknowledgements
We would like to thank Fred Kelcz, MD, JulieAnn Stover, MD, and Shane Rassman, MD, for participating in the reader studies. We appreciate support from the RSNA, GE Healthcare, NIH/NCI P30 CA014520, and the Department of Radiology and Cancer Center at the authors’ institution.References
1. Kuhl CK, Schild HH, Morakkabati N. Dynamic bilateral contrast-enhanced MR imaging of the breast: Trade-off between spatial and temporal resolution. Radiology 2005;236(3):789-800. 2. Lustig M, Donoho D, Pauly JM. Sparse MRI: The application of compressed sensing for rapid MR imaging. Magn Reson Med 2007;58(6):1182-1195. 3. Gamper U, Boesiger P, Kozerke S. Compressed sensing in dynamic MRI. Magn Reson Med 2008;59(2):365-373. 4. Hargreaves BA, Saranathan M, Sung K, Daniel BL. Accelerated breast MRI with compressed sensing. Eur J Radiol 2012;81S1:S54-55. 5. King K, Xu D, Brau AC, Lai P, Beatty PJ, Marinelli L. A new combination of compressed sensing and data driven parallel imaging. Proceedings of the Annual Meeting of ISMRM. Stockholm, 2010. (abstract 4881).Figures