4852
MR- and optical-based multimodal and multiscale protocol for mice colorectal diseases diagnosis1Univ Lyon, INSA‐Lyon, Université Lyon 1, UJM-Saint Etienne, CNRS, Inserm, CREATIS UMR 5220, U1206, Lyon, France, 2Institut de Génomique Fonctionnelle de Lyon, Université de Lyon 1, UMR 5242 CNRS, Ecole Normale Supérieure de Lyon, Lyon, France, 3LTSI; INSERM U642; Université Rennes 1, Rennes, France, 4Hôpital Régional Universitaire de Tours - Service hépato-gastroentérologie, Tours, France
Synopsis
A multimodal and multiscale protocol was defined for the diagnosis of mice colorectal diseases. Based on endoluminal MRI, using dedicated endorectal coils, and optical modalities the protocol was assessed on a mouse model of colorectal cancer for a six-month period. Optical modalities were used for microscopic characterization of the surface of the colon wall where endoluminal MRI was used for in-depth macroscopic characterization and staging of lesions from inflammation to cancer. The protocol was then used to support in vivo MRS signal analysis that was used to assess the biochemical content of various anatomical structures (colon wall, visceral fat…).
PURPOSE
Preclinical trials are often a prerequisite before the transfer to the clinic. To consider the benefit of a novel imaging technique or treatment, preclinical protocols must allow an evaluation at different scales with different sources of contrast. In this abstract, we propose a “generic” protocol combining endoluminal MRI with optical modalities that can be used to evaluate new diagnosis techniques or treatment for endocavitary applications. We have assessed the efficiency and reproducibility of the protocol on a mouse model of colorectal cancer (CRC) during which we have implemented in vivo MR spectroscopy.METHODS
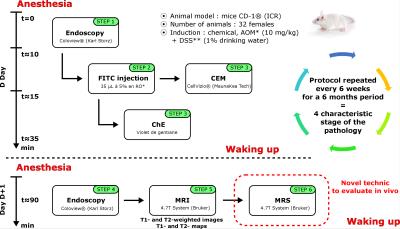
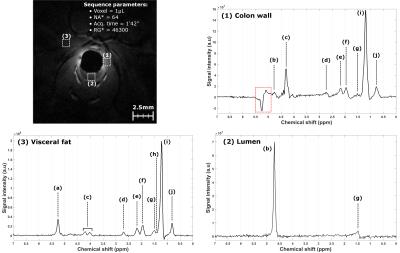
The preclinical protocol combined endoluminal MRI, using dedicated endorectal coils (ERC)1, with optical modalities gathering: conventional endoscopy (CE), chromoendoscopy (ChE) and confocal laser endomicroscopy (CEM), using FITC as contrast agent2. The pathology model of CRC3 was chemically induced on 20 mice, and 12 healthy animals served as control. Each mouse was imaged with all modalities every six weeks for a six-month period. Images were classified by a radiologist regarding the characteristic stage of the development of CRC, as follows: healthy tissues, inflammation, infiltration, and tumors. The timeline of the protocol is represented in Figure 1. The endoluminal MRI acquisition protocol included high spatial resolution T1-weighted images and their associated T1-maps. Finally, we have assessed the potential of in vivo MRS, using ERC and the dedicated protocol, in the characterization of CRC on mice. After first order shim correction, PRESS mono-voxels (TE=16.2ms, TR=1500ms and various sizes from 0.125μL to 4μL acquired in less than 3 minutes), without water suppression, were precisely acquired at different locations (healthy colon walls, visceral fat, inflammatory lesions and tumors) confirmed by a radiologist.RESULTS
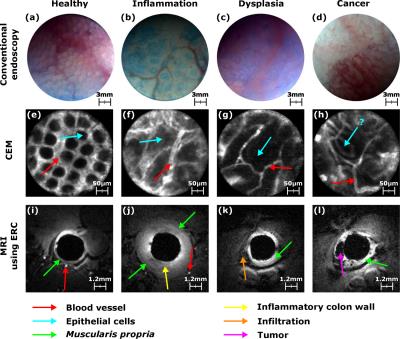
Optical modalities were used to detect and microscopically
characterize CRC lesions at the surface of the colon wall (CW) – see Figure 2. CE is used to
depict suspicious areas where ChE and CEM are used to characterize the vascular
network distribution surrounding the epithelial cells (see Figure 2). Endoluminal
MRI (T1-weighted images) was performed for macroscopic in-depth characterization
and staging (see Figure 2).
At each stage of the pathology, in vivo
MRS was evaluated. Characteristic spectra were acquired and exhibit various
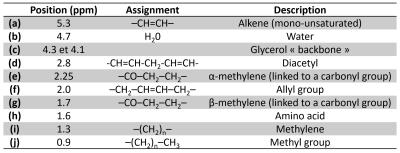
biochemical contents following the location (see Figure 3 and Table 1).DISCUSSION
Based on this multiscale, multicontrast and multimodality protocol, the entire follow-up of the pathology can be performed. Colocalization between CE, ChE and CEM images improve the diagnosis of lesions by characterizing the macroscopic aspect to the microscopic development of abnormal cells in the epithelium. Some of flat lesions detected in CE were labeled at inflammation showing no aggressive behavior, but, when imaged with endoluminal MRI, these lesions appear to be more severe than expected drastically changing the short-term prognosis of the animal. This robust protocol to depict, characterize and stage colorectal abnormalities with different sources of contrast was then used to evaluate in vivo MRS. Based on images provided by both modalities, spectra were acquired in specific regions. Using ERC, acquisition times were kept as short as possible (less than 3 minutes) and prescribed volumes ranged from 0.12µL to 1µL to precisely fit with small anatomical structures. Achieved scan time and analysis volume made in vivo MRS suitable for clinical applications. Spectra acquired in the colon wall show similar line shape contributions to spectra acquired in visceral fat. A new longitudinal study will help to evaluate the benefit of this technique for the biochemical characterization of suspicious areas located. Nevertheless, for the first time reported in the literature, in vivo MRS was performed on mice colon and rectum thanks to ERC.CONCLUSION
This multiscale and multicontrast protocol has demonstrated its efficiency and reliability to depict, to characterize and to stage the different patterns of a pathology through time. The protocol can also include MRS for CRC examination and could be used for other applications such as the study of the intestinal microbiota.Acknowledgements
This work was supported by the LABEX PRIMES (ANR-11-LABX-0063) of Université de Lyon, within the program "Investissements d'Avenir" (ANR-11-IDEX-0007) operated by the French National Research Agency (ANR). This work was performed on the platform PILoT, member of France Life Imaging network (grant ANR-11-INBS-0006).References
1. Dorez, H. et al. Endoluminal high-resolution MR imaging protocol for colon walls analysis in a mouse model of colitis. Magn. Reson. Mater. Phys. Biol. Med. 1–13 (2016). doi:10.1007/s10334-016-0539-2
2. Waldner, M. J., Wirtz, S., Neufert, C., Becker, C. & Neurath, M. F. Confocal laser endomicroscopy and narrow-band imaging-aided endoscopy for in vivo imaging of colitis and colon cancer in mice. Nat. Protoc. 6, 1471–1481 (2011).
3. Tanaka, T. et al. A novel inflammation-related mouse colon carcinogenesis model induced by azoxymethane and dextran sodium sulfate. Cancer Sci. 94, 965–973 (2003).
Figures