4850
Magnetic Resonance Spectroscopy of the Testis under Ischemic Condition1Division of Functional Imaging, National Cancer Center, Kashiwa, Japan, 2National Institute for Environmental Studies, Tsukuba, Japan
Synopsis
Testicular ischemia is an acute disorder, which requires an accurate diagnosis whether the testis function is reversible or irreversible at the time of presentation; however, diagnostic test that predicts the functional reversibility of the ischemic testis has yet been established. This paper reports temporal metabolite changes for up to 24 hours after the onset of experimental testicular ischemia by using a 9.4-tesla magnetic resonance spectroscopy. We found the reduction in creatine levels in the testis under prolonged ischemic condition; hence, the alteration in creatine levels could be a possible metabolite marker that indicates the functional reversibility of the ischemic testis.
INTRODUCTION
Testicular ischemia is one of the pediatric emergencies, which requires
an accurate and timely diagnosis to save the testis.1 This
disorder is caused by torsion of the spermatic cord, thus surgical detorsion is
the treatment of choice especially within 12 hours of onset, during which
testicular damage is reportedly reversible. However, the diagnosis is usually
made later; thus, surgeons have to make a decision whether they repair the torsion
(salvage the testicle) or excise the damaged testicle without any clinical marker
that predicts the reversibility of testicular function. Magnetic resonance
spectroscopy (MRS)-detectable metabolites in the testis can provide valuable
information on testicular function,2-4 therefore,
MRS is capable of detecting metabolite signatures that indicate reversible or
irreversible testicular damages. While finding such metabolite signatures is the
ultimate goal, the purpose of this study was to examine temporal
metabolite changes in the testis under ischemic condition by using MRS because it
has yet been fully investigated in the literature.METHODS
The Institutional Animal Care and Use Committee approved this animal experiment. We used 15 Wistar rats and produced testicular ischemia by ligating the left testicular vasculatures. The right testes served as controls. We excised and immediately freeze-clamped the bilateral testicles at 1, 4, 6, and 12 hours (n=2 at each time point) as well as 24 hours (n=7) after ligation and performed perchloric acid extraction to obtain testis extracts. We lyophilized the extracts, reconstituted them with deuterium oxide, added 5 µmole 3-(trimethylsilyl)propionic-2,2,3,3-d4 acid as an external standard, and acquired NMR spectra of the extract by using a 400-MHz spectrometer and solvent-suppressed 1D pulse sequence (zgpr; BrukerBiospin, Germany). In 4 rats, we also performed in vivo MRS measurement of the testis at 24 hours after ligation by using a 9.4-tesla scanner (BioSpec 94/20USR, BrukerBiospin) and stimulated echo acquisition mode (STEAM, TR/TM/TE = 1500/10/3 ms) sequence. We assigned metabolite peaks referencing previous reports2, 5, 6 and measured integrals of methyl peaks of lactate, N-methyl peaks of creatine, and N-trimethyl peaks at around 3.2-3.3 ppm at each time point by using a manufacturer-supplied software (TopSpin, BrukerBiospin). We examined the differences in these metabolite levels between the 24-hr ischemic and control testes by using Tukey's test.RESULTS
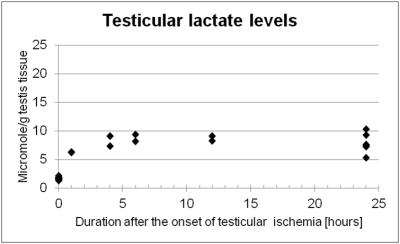
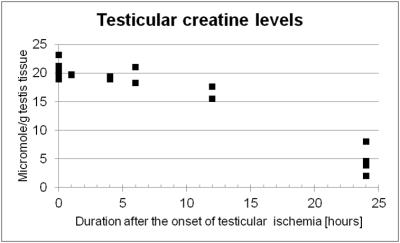
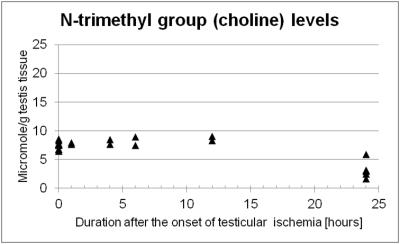
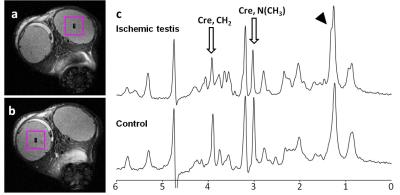
Lactate levels in the ischemic testis were elevated at one hour after ligation and showed significantly higher levels than the control testis at 24 hours (7.82 ± 1.74 vs. 1.67 ± 0.21 µmole/g testis tissue, P <0.001, Figure 1). Noticeably, creatine levels in the ischemic testis were significantly reduced at 24 hours (4.54 ± 1.95 vs. 20.61 ± 1.38 µmole/g testis tissue, P <0.001, Figure 2). N-trimethyl groups also showed significantly lower levels in the 24-hour ischemic testis (3.22 ± 1.44 vs. 7.77 ± 0.55 µmole/g testis tissue, P <0.001, Figure 3). In vivo MRS (Figure 4) showed the reduction in the N-methyl peak of creatine at 3.03 ppm relative to the N-trimethyl peak (so called total choline) at 3.21 ppm. The ratio of the creatine to the total choline peaks was 0.60 ± 0.09 in the 24-hour ischemic testis and 1.03 ± 0.24 in the control testis (P <0.05, t-test). Elevated lactate peaks were obscured by large lipid peaks at around 1.3 ppm.2DISCUSSION
The elevation of lactate levels in the ischemic testis is probably caused by anaerobic glycolysis occurring under hypoxic condition. Our MRS data showed high levels of creatine in the normal testis as reported previously.5, 7 Testis tissues, more specifically Sertoli cells, reportedly synthesize creatine molecules from guanidinoacetate and s-adenosylmethionine utilizing an enzyme, guanidinoacetate methyltransferase, and secrete the molecules to the seminiferous tubular lumen where testicular sperm utilizes creatine molecules as high-energy phosphate carriers.8 We observed creatine levels significantly reduced in the testis under prolonged ischemic condition which resulted in irreversible testicular damages histologically (i.e. testicular infarction).9 The precise mechanism of this creatine reduction is under investigation; however, the molecules possibly leak out from the tubular lumen to the blood stream as Gray and coworkers previously reported creatinemia after testicular ischemia in rats and they also demonstrated that orchiectomy avoided the increase of blood creatine levels.10 In addition, the reduction in N-trimethyl proton (including choline compounds' proton) levels in the prolonged ischemic testis could indicate that membrane turnover of testicular cells is disrupted.CONCLUSION
We examined temporal metabolite changes in the testis under ischemic condition by using MRS and found that the reduction in creatine levels was indicative of prolonged ischemic damage of the testis.Acknowledgements
This study was supported by grants from Foundation for Promotion of Cancer Research in Japan and Suzuki Foundation for Urological Medicine to MY. Authors thank Dr. Shusei Hamamichi for English proof reading.References
1. DaJusta DG, Granberg CF, Villanueva C, Baker LA. Contemporary review of testicular torsion: new concepts, emerging technologies and potential therapeutics. J Pediatr Urol 2013; 9: 723-730.
2. Yamaguchi M, Mitsumori F, Watanabe H, Takaya N, Minami M. In vivo localized 1H MR spectroscopy of rat testes: stimulated echo acquisition mode (STEAM) combined with short TI inversion recovery (STIR) improves the detection of metabolite signals. Magn Reson Med 2006; 55: 749-754.
3. Aaronson DS, Iman R, Walsh TJ, Kurhanewicz J, Turek PJ. A novel application of 1H magnetic resonance spectroscopy: non-invasive identification of spermatogenesis in men with non-obstructive azoospermia. Hum Reprod 2010; 25: 847-852.
4. Mallidis C, Green BD, Rogers D, Agbaje IM, Hollis J, Migaud M, Amigues E, McClure N, Browne RA. Metabolic profile changes in the testes of mice with streptozotocin-induced type 1 diabetes mellitus. Int J Androl 2009; 32: 156-165.
5. Navon G, Gogol E, Weissenberg R. Phosphorus-31 and proton NMR analysis of reproductive organs of male rats. Arch Androl 1985; 15: 153-157.
6. Griffin JL, Troke J, Walker LA, Shore RF, Lindon JC, Nicholson JK. The biochemical profile of rat testicular tissue as measured by magic angle spinning 1H NMR spectroscopy. FEBS Lett 2000; 486: 225-229.
7. Lee HJ, Fillers WS, Iyengar MR. Phosphocreatine, an intracellular high-energy compound, is found in the extracellular fluid of the seminal vesicles in mice and rats. Proc Natl Acad Sci U S A 1988; 85: 7265-7269.
8. Lee H, Kim JH, Chae YJ, Ogawa H, Lee MH, Gerton GL. Creatine synthesis and transport systems in the male rat reproductive tract. Biol Reprod 1998; 58: 1437-1444.
9. Yamaguchi M, Mitsumori F, Watanabe H, Takaya N, Minami M. Visualization of seminiferous tubules in rat testes in normal and diseased conditions by high-resolution MRI. Magn Reson Med 2009; 62: 637-644.
10. Gray J, Nicholson JK, Creasy DM, Timbrell JA. Studies on the relationship between acute testicular damage and urinary and plasma creatine concentration. Arch Toxicol 1990; 64: 443-450.
Figures