4849
Water diffusion MRI as a biomarker of fetal lung development1Center for Pulmonary Imaging Research, Cincinnati Children’s Hospital Medical Center, Cincinnati, OH, United States, 2Department of Physics, University of Cincinnati, Cincinnati, OH, United States, 3Advanced Imaging Research Center, Oregon Health & Science University, Portland, OR, United States, 4Department of Physics, Washington University in St. Louis, St. Louis, MO, United States, 5Pulmonary Biology, Cincinnati Children’s Hospital Medical Center, Cincinnati, OH, United States, 6Department of Radiology, CCHMC, Cincinnati, OH, United States
Synopsis
In the developing fetal lung of both humans and rhesus macaques, the amount of interstitial tissue decreases during the transition from the canalicular to saccular stage. We hypothesize that this change corresponds to a decrease in restricted 1H diffusion in fetal lungs. 17 rhesus fetal lungs (in-vivo and ex-vivo) were imaged at gestation days 83-85, 110, and 133-135 with diffusion-weighted MRI. The apparent diffusion coefficients (ADCs, normalized by free-diffusion) significantly increased with gestational age for both in-vivo and ex-vivo experiments. These results demonstrate that ADC in the fetal lung can be used as a biomarker for the degree of alveolarization.
Introduction
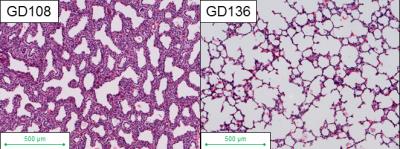
Water diffusion-weighted imaging (DWI) is often used to characterize brain development and injury, typically because of the sensitivity of the technique to restricted diffusion (and/or anisotropy) within cellular spaces. The developing lung also experiences significant cellular changes in utero; the number of alveolated (open) air-spaces that are fluid-filled in utero increases with development, and consequently, water diffusion might be expected to become less restricted throughout gestation. Past attempts to demonstrate this have had conflicting results1,2,3. The fetal rhesus macaque monkey lung is a promising model for human fetal lung development, due to the similarities of developmental stages and timing between the macaque and human3. There are four developmental stages of the lung in both the macaque and human, with clear structural and cellular changes at each stage. In the macaque, these correspond to the following timepoints over the 168 day gestational term: embryonic stage (gestation day [GD] 21-55); pseudo-glandular stage (GD 56-80); canalicular stage (GD80-130); and saccular stage (GD131-165)4,5. As the alveolar spaces expand during transition from the canalicular to the saccular stage, the amount of interstitial tissue decreases, which leads to decreased volume density of cells (Figure 1) and could lead to less restricted diffusion. In this study we investigated the changes in restrictions to water diffusion by imaging both in-vivo and ex-vivo fetal macaque lungs with 1H DWI MRI, using apparent diffusion coefficient (ADC) maps to determine the degree of correspondence with alveolarization.Methods
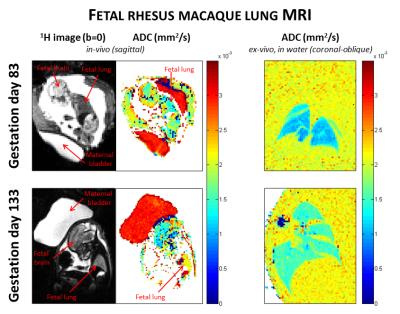
Our examinations were conducted using 17 rhesus macaque fetal lungs at gestation days 83-85 (N=4), 110 (N=5) and 133-135 (N=8) from the Oregon National Primate Research Center. During in-vivo experiments, 16 fetal macaque lungs were imaged in utero with a spin-echo EPI DWI sequence on a 3T Siemens MRI system (9 b-values from 0-500 s/mm2, slice thickness = 2 mm, TR = 4200 ms, TE = 150 ms, FA = 90o, in-plane resolution = 0.5 mm) with the maternal bladder evident for measurement of temperature-matched unrestricted diffusion. Image registration was performed on the in-vivo images using the offline software Advanced Normalization Tools (ANTs)6 to remove bulk motion artifacts. After sacrifice the fetal lungs were fixed at 20 cm H2O inflation pressure with 4% paraformaldehyde doped with 2.5mM Gd contrast agent. These ex-vivo lungs were imaged using a 2D gradient-echo DWI sequence on a 7T Bruker scanner (9 b-values from 0-500 s/mm2, slice thickness = 1 mm, TR = 31.6 ms, TE = 12.8 ms, FA = 20o, in-plane resolution = 0.55 mm), with an adjacent water phantom for comparison. ADC maps of both in-vivo and ex-vivo fetal lungs were generated from a mono-exponential decay model. Mean restricted values for the ADC were calculated in regions of interest (ROIs) within both in-vivo and ex-vivo lungs, while mean unrestricted water ADC values were calculated in bladder ROIs (in-vivo) and water ROIs (ex-vivo). Unrestricted ADC values (bladder or water phantom) were used to normalize the restricted lung ADC values.Results
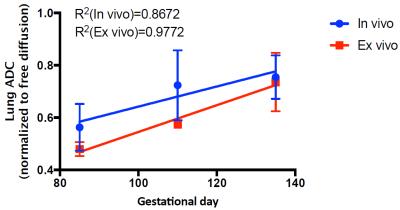
Representative fetal lung images and ADC maps at the canalicular stage (GD83) and at the saccular stage (GD133) are shown in Figure 2. The first column shows b=0 images to demonstrate how the lung was identified, and the second and third columns present in-vivo and ex-vivo ADC maps, respectively. These results indicate that the ADC increases across these two stages of lung development. Figure 3 demonstrates the normalized ADC significantly increasing with gestational age for both in-vivo and ex-vivo results. The mean normalized lung ADCs were as follows: GD83-85: 0.563 ± 0.090 in-vivo and 0.480 ± 0.027 ex-vivo; GD110: 0.724 ± 0.134 in-vivo and 0.574 ex-vivo; GD133-135: 0.755 ± 0.083 in-vivo and 0.736 ± 0.112 ex-vivo. Differences in ADC between GD83-85 and GD133-135 were statistically significant (p = 0.0008 in-vivo and p = 0.0017 ex-vivo, from an unpaired t-test), but no significant differences were seen between the intermediate-stage lungs at GD110 and either the earlier or later stages of development.Discussion and Conclusions
This work demonstrates that the normalized 1H ADC can be used as a biomarker of fetal lung development in the rhesus macaque, particularly between early canalicular and saccular stages. Due to the similarity of the cellular development, airway architecture, and developmental timeline between rhesus macaque lungs and human lungs, this method can easily be translated to human studies.Acknowledgements
The authors thank the following sources for research funding and support: NIH R01 AA021981, and the Oregon National Primate Research Center core grant P51OD011092.References
1. Balassy C, Kasprian G, Brugger P, et al. Diffusion-weighted MR imaging of the normal fetal lung. Eur Radiol. 2008;18(4):700-706.
2. Lee W, Krisko A, Shetty A, et al. Noninvasive Fetal Lung Assessment Using Diffusion Weighted Imaging. Ultrasound Obstet Gynecol. 2009;34(6):673–677.
3. Afacan O, Gholipour A, Mulkern R, et al. Fetal lung apparent diffusion coefficient measurement using diffusion-weighted MRI at 3 Tesla: Correlation with gestational age. J Magn Reson Imaging. 2016;doi: 10.1002/jmri.25294.
4. Kerr G, Couture J, Allen J. Growth and development of the fetal rhesus monkey. VI. Morphometric analysis of the developing lung. Growth, 1975;39:67-84.
5. Van Winkle L, Murphy S, Boetticher M, et al. Fetal Exposure of Rhesus Macaques to Bisphenol A Alters Cellular Development of the Conducting Airway by Changing Epithelial Secretory Product Expression. Environ Health Perspect. 2013;121(8):912-918.
6. Avants A, Tustison N, Song G, et al. Reproducible Evaluation of ANTs Similarity Metric Performance in Brain Image Registration. Neuroimage. 2011;54(3):2033-2044.
Figures