4848
Assessing placenta injury with anatomical and IVIM-diffusion MRI in a mouse model of intrauterine inflammation1Radiology, Johns Hopkins University School of Medicine, BALTIMORE, MD, United States, 2Gynecology and Obstetrics, Johns Hopkins University School of Medicine, BALTIMORE, MD, United States
Synopsis
In this study, we investigated the placenta anatomy and function in a mouse model of intrauterine inflammation, using T2-weighted MRI and introvoxel incoherent motion (IVIM) MRI to measure placental perfusion. The high-resolution T2-weighted images demonstrated altered placenta anatomy in response to the acute inflammatory injury, which agreed with the histological measurements. IVIM of the mouse placenta was acquired with diffusion-weighted echo-planar imaging in a reduced field-of-view. The pseudo-diffusion fraction (f) and coefficient (D*) fitted from IVIM model indicated reduced perfusion volume (f) and velocity (f·D*) in the injured placentas compared to the shams.
Purpose
The placenta is a vital organ in supporting the developing fetus, and placenta insufficiency can lead to severe complications such as fetal growth restriction and preterm birth. MRI plays a unique role in assessing the placenta anatomy and function, including the blood flow dynamics. In addition to the conventional perfusion MRI measurements1,2, intra-voxel incoherent motion (IVIM) imaging3 provides useful information about blood flow in the capillaries and small vessels4,5, which has been previously used to measure placenta perfusion in human and animal models6-10. In this study, we investigated the capability of IVIM technique in examining acute changes in the mouse placenta in response to intrauterine inflammation11, along with the structural assessment by high-resolution T2-weighted imaging.Methods
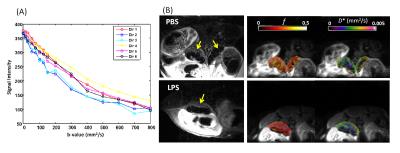
Intrauterine inflammation was induced in pregnant CD1 mice (average litter size of eleven) at embryonic day 17, by intrauterine injection of Lipopolysaccharide (LPS) as opposed to the shams that received Phosphate-buffered saline (PBS). Animals were imaged at 6hrs after the surgery, on an 11.7T horizontal Bruker scanner. An eight-channel rat body array coil was used for whole-body T2-weighted MRI, which were acquired at FOV of 40x40 mm, in-plane resolution of 0.16x0.16 mm, 60 slices with 0.5mm thickness, TE/TR = 37.5/6000ms, and eight spin-echoes. IVIM imaging was performed using a 15mm planner surface coil placed on the mouse abdomen to restrict the FOV and to increase SNR. Data were acquired with a single-shot EPI sequence at FOV of 28x24 mm, in-plane resolution of 0.3x0.3 mm, 20 slices with 1mm thickness, TE/TR =28.2/5000ms, 16 b-values ranging from 10 to 800 s/mm2, six directions, and scan time of 16 min with respiration triggers. IVIM fitting was performed following a two-step fitting procedure, as described in (6), based on the bi-exponential model S/S0 = f ∙D* + (1-f) ∙D, where f, D*, and D are the pseudo-diffusion fraction, pseudo-diffusion coefficient, and diffusion coefficient, respectively.Results
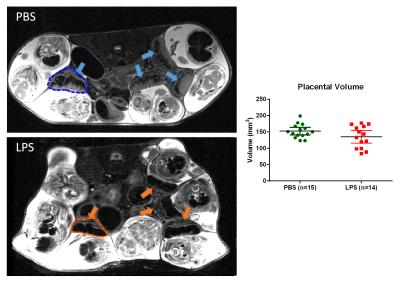
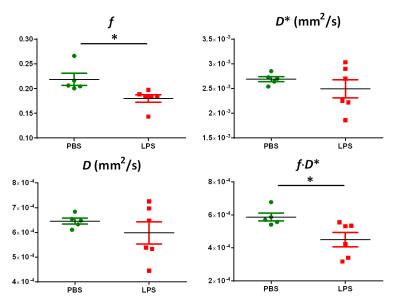
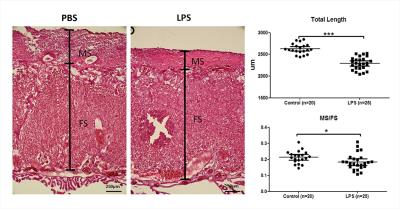
High-resolution T2-weighted images demonstrated the morphological changes in placenta at 6hrs after LPS challenge, compared to the PBS mice (Figure 1). Placentas were manually delineated on the T2-weighted images, and the volumes were obtained. The PBS group showed slightly higher placenta volumes (152±12 mm3, n=14) compared to the LPS group (135±33 mm3, n=15) with a p-value of 0.12 by unpaired t-test with Welch’s correction. Diffusion MRI signals followed a bi-exponential decay in all directions (Figure 2A), based on which, f and D* values were derived from the IVIM model. Figure 2B illustrated the f and D* measurements mapped onto the non-diffusion weighted images of a PBS and a LPS mouse. The measure of f, which relates to blood volume5, showed a significant reduction in the LPS-exposed placentas (0.18±0.01, n=6) compared to that in the PBS group (0.22±0.01, n=5) with a p-value of 0.021; and the measure of f ∙D*, which relates to blood velocity5, also showed significant lower values in the LPS group (4.5±0.4 x10-4 mm2/s) compared to the PBS group (5.8±0.2 x10-4 mm2/s) (p=0.028); whereas the D* and D values did not show significant difference between the two groups (Figure 3). Nissl staining showed significantly reduced total length in the LPS-exposed placentas (n=25) compared to the PBS-exposed ones (p<0.001, n=20). The reduction was more severe in the maternal side of the placenta compared to the fetal side, leading to a decreased MS-to-FS length ratio in the LPS group(Figure 4).Discussion and Conclusion
Placenta imaging is a promising tool for structural and functional assessments of placenta sufficiency, which however, is challenging due to the inevitable fetal and maternal motion, low SNR resulted from the fast T2 and T2* decay of blood, and air/fat/tissue inhomogeneity in the complex placental environment. In this study, we took a unique approach of using restricted FOV for IVIM imaging with a surface coil. With the restricted FOV, the readout time and echo time of a single-shot EPI can be reduced, to compensate for the short T2 relaxation of blood in the placenta and the field inhomogeneity. Our results demonstrated that both the f and f ∙D* measurements were able to capture the placenta dysfunction in response to acute intrauterine inflammation, with a relatively higher sensitivity compared to volumetric measures. The histological results supported the structural MRI observation. For example, the maternal side (layer of bright issue in T2w) demonstrated a larger degree of thinning compared to the fetal side (dark tissue in T2w). A two-compartment analysis of the T2 and IVIM data will be needed to explore the relative changes of the fetal and maternal parts, and the sample size needs to be increased to strengthen the findings.Acknowledgements
This work was made possible by the following funding supports: R21 NS098018 (DW) and K08HD073315 (IB).References
1. Williams DS, Detre JA, Leigh JS, Koretsky AP. Magnetic-Resonance-Imaging of Perfusion Using Spin Inversion of Arterial Water. P Natl Acad Sci USA 1992;89(1):212-216.
2. Smith AM, Grandin CB, Duprez T, Mataigne F, Cosnard G. Whole brain quantitative CBF and CBV measurements using MRI bolus tracking: Comparison of methodologies. Magnet Reson Med 2000;43(4):559-564.
3. Lebihan D, Breton E, Lallemand D, Grenier P, Cabanis E, Lavaljeantet M. Mr Imaging of Intravoxel Incoherent Motions - Application to Diffusion and Perfusion in Neurologic Disorders. Radiology 1986;161(2):401-407.
4. Iima M, Reynaud O, Tsurugizawa T, Ciobanu L, Li JR, Geffroy F, Djemai B, Umehana M, Le Bihan D. Characterization of Glioma Microcirculation and Tissue Features Using Intravoxel Incoherent Motion Magnetic Resonance Imaging in a Rat Brain Model. Invest Radiol 2014;49(7):485-490.
5. Federau C, O'Brien K, Meuli R, Hagmann P, Maeder P. Measuring brain perfusion with intravoxel incoherent motion (IVIM): Initial clinical experience. J Magn Reson Imaging 2014;39(3):624-632.
6. Solomon E, Avni R, Hadas R, Raz T, Garbow JR, Bendel P, Frydman L, Neeman M. Major mouse placental compartments revealed by diffusion-weighted MRI, contrast-enhanced MRI, and fluorescence imaging. P Natl Acad Sci USA 2014;111(28):10353-10358.
7. Moore RJ, Issa B, Tokarczuk P, Duncan KR, Boulby P, Baker PN, Bowtell RW, Worthington BS, Johnson IR, Gowland PA. In vivo intravoxel incoherent motion measurements in the human placenta using echo-planar imaging at 0.5 T. Magnet Reson Med 2000;43(2):295-302.
8. Moore RJ, Strachan BK, Tyler DJ, Duncan KR, Baker PN, Worthington BS, Johnson IR, Gowland PA. In utero perfusing fraction maps in normal and growth restricted pregnancy measured using IVIM echo-planar MRI. Placenta 2000;21(7):726-732.
9. Moore RJ, Ong SS, Tyler DJ, Duckett R, Baker PN, Dunn WR, Johnson IR, Gowland PA. Spiral artery blood volume in normal pregnancies and those compromised by pre-eclampsia. Nmr Biomed 2008;21(4):376-380.
10. Alison M, Chalouhi GE, Autret G, Balvay D, Thiam R, Salomon LJ, Cuenod CA, Clement O, Siauve N. Use of Intravoxel Incoherent Motion MR Imaging to Assess Placental Perfusion in a Murine Model of Placental Insufficiency. Invest Radiol 2013;48(1):17-23.
11. Burd I, Bentz AI, Chai J, et al. Inflammation-induced preterm birth alters neuronal morphology in the mouse fetal brain. J Neurosci Res 2010;88:1872–1881.
Figures