4847
Hepatocellular carcinoma (HCC) screening with contrast-enhanced liver MRI: View-sharing artifact reduction with retrospective compressed sensing reconstruction1Radiology, Stanford University, Stanford, CA, United States, 2Electrical Engineering and Radiology, Stanford University, Stanford, CA, United States, 3Radiology, Veteran Affairs Palo Alto Health Care System
Synopsis
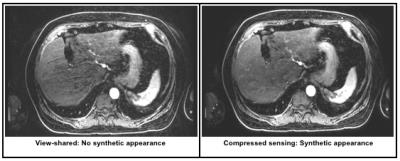
Hepatocellular carcinoma (HCC) screening is a common indication for contrast-enhanced liver MRI. Dynamic contrast-enhanced (DCE) acquisitions often utilize view-sharing (VS) to optimize spatiotemporal resolution, but MRI can often be degraded by respiratory motion. VS introduces temporal blurring of high spatial frequencies that propagate coherent motion artifacts across phases. Compressed sensing (CS) can reduce the need for VS by recovering missing k-space data from pseudo-random undersampling, thus potentially reducing temporal blurring while maintaining spatial resolution. CS results in greatly reduced ghosting artifacts despite a more synthetic appearance.
Introduction
Hepatocellular carcinoma (HCC) screening is one of the most common indications for contrast-enhanced liver MRI. Yet, liver imaging is often degraded by coherent ghosting artifacts that arise from incomplete suspension of respiration. These artifacts make interpretation challenging and can undermine sensitivity of MRI, which is otherwise the best detection method for HCC.1
Diagnostic criteria for HCC are defined by the Organ Procurement and Transplantation Network/United Network for Organ Sharing (OPTN/UNOS) and used extensively in radiologic reporting at major transplant centers.2 As outlined by OPTN/UNOS, the crux of diagnosing HCC by MRI relies on a high-quality dynamic contrast-enhanced (DCE) acquisition with optimal imaging of the hepatic arterial phase. Inherent in DCE is the opportunity to capture multiple phases of enhancement after contrast injection.
DCE of the liver requires 3D high spatiotemporal resolution T1-weighted imaging. However, maximizing temporal resolution is a competing goal with spatial resolution. View-sharing (VS) improves temporal resolution through rapid and frequent acquisition of central k-space data and interleaved acquisition of pseudo-randomly segmented outer k-space data.3 Outer k-space data is then shared between temporal phases allowing preservation of spatial resolution at a cost of temporal blurring.
Iterative compressed sensing (CS) methods that require pseudo-random sampling can exploit inherent spatiotemporal sparsity and reconstruct images with less VS data, providing retrospectively a shorter “temporal footprint” while leaving the acquisition unchanged.4,5 We hypothesized that an iterative CS reconstruction of reduced VS data would be superior to VS in image quality, anatomic delineation, and detection of HCC.
Methods
This was an IRB-approved, HIPAA-compliant retrospective study of DCE comparing VS to CS in patients who received MRI for HCC screening. Patients with treated HCC were excluded.
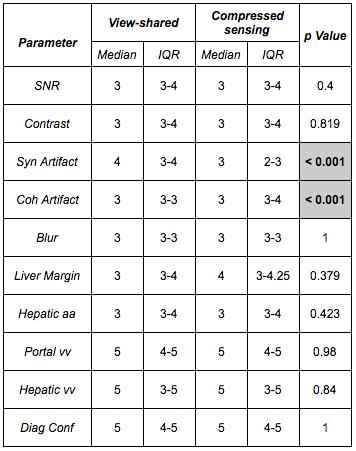
Two radiologists independently assessed VS and CS images for image quality (1-4; inferior-superior) and anatomic delineation (1-5; inferior-superior). OPTN/UNOS criteria were used for HCC diagnosis. Diagnostic confidence in lesion detection was also scored (1-5; low-high). VS and CS series were anonymized and randomized, resulting in blinded assessments. Subsequently, side-by-side blinded evaluations were performed (-3 to 3; 0 implying equivalence).
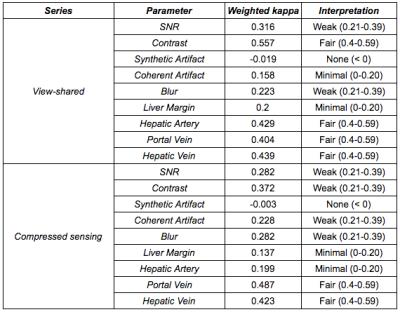
Weighted Cohen’s kappa was used to evaluate inter-reader agreement. Wilcoxon signed rank tests were performed for paired, nonparametric analyses with a Bonferroni-Holm correction for multiple comparisons. McNemar’s test assessed for differences in frequency of lesion detection.
Results
The final cohort of 33 patients had an average age of 61 ± 10 years, with 20 males.
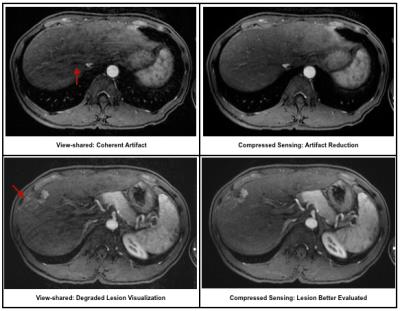
Inter-reader agreement was more substantial for general image quality metrics than anatomic delineation (Table 1). CS demonstrated significantly less coherent artifact than VS (Table 2; Figure 1). A synthetic appearance on CS was present, an expected finding using an iterative reconstruction (Figure 2).
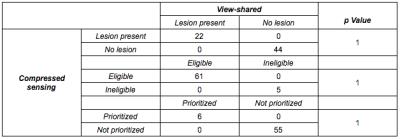
Regarding perceived diagnostic confidence in interpretation, no significant difference was noted between VS and CS. More importantly, frequency of lesion detection did not differ by technique; every case of HCC on VS was detected on CS. This implies comparability of CS to VS for the most vital aspect of the exam: detection of HCC (Table 3). This remained true when stratified by size. Similarly, no change in eligibility or prioritization for transplantation would have occurred using CS.
Side-by-side evaluations revealed a significant preference for CS over VS when displayed side-by-side (p = 0.005).
Discussion
We demonstrated that CS can significantly reduce coherent artifacts, and readers aesthetically preferred CS over VS. One often cited imperfection of iterative reconstructions is a resultant synthetic appearance. However, this is not unanimously viewed as undesirable, especially among newly-trained radiologists who have greater exposure to iterative reconstructions in the context of computed tomography.
Importantly, we demonstrated CS was manifestly reliable in detection of HCC. Similarly, no change in eligibility or prioritization for transplantation would have occurred through use of CS.
Conclusion
Retrospective use of CS can add robustness to a clinical DCE protocol using randomized sampling and VS. Expanded application of this technique beyond HCC will firmly establish its clinical benefit.Acknowledgements
This work was in part supported by NIH grant P41 EB015891 and R01 EB009055. The authors would also like to thank the support of GE Healthcare.References
1. Pitton MB, Kloeckner R, Herber S, Otto G, Kreitner KF, Dueber C. MRI versus 64-row MDCT for diagnosis of hepatocellular carcinoma. World J Gastroenterol. 2009;15:6044-6051.
2. Rosenkrantz AB, Campbell N, Wehrli N, Triolo MJ, Kim S. New OPTN/UNOS classification system for nodules in cirrhotic livers detected with MR imaging: effect on hepatocellular carcinoma detection and transplantation allocation. Radiology. 2015; 274:426-433.
3. Saranathan M, Rettmann DW, Hargreaves BA, Clarke SE, Vasanawala SS. Differential subsampling with Cartesian ordering (DISCO): a high spatio-temporal resolution Dixon imaging sequence for multiphasic contrast enhanced abdominal imaging. J Magn Reson Imaging. 2012; 35:1484-1492.
4. Liang ZP. Spatiotemporal imaging with partially separable functions. IEEE International Symposium on Biomedical Imaging: From Nano to Macro. 4th IEEE; 2007.
5. Levine E, Daniel B, Vasanawala SS, Hargreaves B, Saranathan M. 3D Cartesian MRI with compressed sensing and variable view sharing using complementary Poisson-disc sampling. Magn Reson Med. 21 April 2016. [Epub ahead of print]
Figures