4846
Semiautomatic determination of arterial input function in breath-hold DCE-MRI of the abdomen1University of Alabama at Birmingham, Birmingham, AL, United States, 2University of Alabama at Birmingham
Synopsis
We developed a semiautomatic technique to determine the arterial input function (AIF) in breath-hold DCE-MRI of the abdomen. The error in AIF was significantly reduced by tracking the motion of aorta. Also, we confirmed that the semiautomatic segmentation of the aortic region can reduce error in AIF induced by manual segmentation up to 15%.
Purpose: Dynamic contrast-enhanced magnetic resonance imaging (DCE-MRI) is a non-invasive method for tissue perfusion assessment. Quantitative DCE-MRI has had limited success for abdominal applications mainly due to breathing associated organ motion. One approach to address this concern is using a repeated breath-hold method rather than free breathing (1, 2). For each breath hold, however, the abdominal aorta may be displaced from the initial position causing error in arterial input function (AIF), when AIF is manually determined. AIF is essential for calculation of perfusion parameters in quantitative DCE-MRI (3). The purpose of this study was to develop a semiautomatic segmentation technique of the abdominal aorta, tracking its motion during breath-hold DCE-MRI. We also examined the amount of error induced by the manual segmentation.
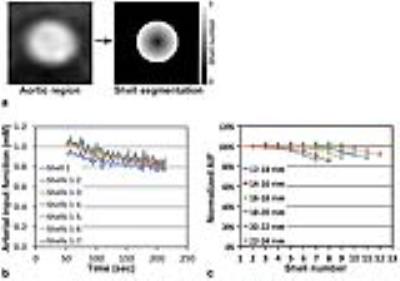
Methods: DCE-MRI was applied for 24 patients having abdominal cancers prior to therapy initiation. All patients were imaged on a 3T MR scanner (Philips Achieva). Variable flip angle approach (5°, 10° and 15°) was utilized for T1 mapping (4). DCE-MRI was conducted with a three-dimensional (3D) fast spoiled turbo gradient echo sequence (THRIVE) with the following parameters: TR/TE = 5/2.3 ms, NEX=1, thickness/gap = 6/0 mm, matrix = 192/154 (interpolated to 256×256), SENSE factor = 2, flip angle = 15°, and the number of slices = 10. DCE-MRI continued for 4.2 minutes with 2.1 seconds temporal resolution (120 acquisitions). Gadoteridol (0.1 mmol/kg) was infused intravenously at 30 seconds after starting DCE-MRI, and flushed with 20-ml saline (2 ml/s). Patients were instructed to hold breath after maximal inhalation, and repeat as needed to full inspiration. Figure 1 shows the procedure of semiautomatic segmentation of the abdominal aorta. When a pixel within the aortic region was manually selected, a cropped image (4×4 cm) containing the aorta was created (Fig. 1a) and interpolated (Fig. 1b). The aortic region was segmented using Otsu’s method (5) (Fig. 1c), and a circle fitting to the segmented image was found (Fig. 1d), and used as the region of interest (ROI) (Fig. 1e). The ROI was determined at each time frame, and the trace of the aorta displacement was quantified. The ROI was further segmented into multiple 1-mm iso-distance shells, and the AIF change according to the decrease of the ROI size was examined.
Results: Figure 2a shows the motion of a representative abdominal aorta after contrast enhancement. Each circle represents the edge of the segmented aortic region at each time frame. Color scale represents the number of overlapped circles. Figure 2b shows the trace of the center position of the aorta shown in Fig. 2a. Figure 2c shows the box plots of the maximum and mean displacements of the aorta from the initial position, when all DCE-MRI slices were analyzed. Figure 3 shows a representative AIF after the second peak signal and the best fitting curve (a) without and (b) with motion tracking. The fitting curve was made by the sequential process of median filtering (3 window) and the 3rd-order polynomial curve fitting. The root mean square error (RMSE) of the AIF from the fitting curve was calculated. Figure 3c shows a box plot of RMSE change (%) after motion tracking. RMSE was significantly decreased by motion tracking (p<0.0001). Figure 4a shows a representative abdominal aorta and its segmentation into seven 1-mm iso-distance shells. Figure 4b shows the AIF change according to the ROI size after the second peak signal. Shell 1 is the core shell, and Shell 7 is the outmost shell. When all seven shells (shells 1-7) were included in the ROI, the magnitude of AIF was lowest, but it increased when the ROI was reduced (that is, fewer shells were included in the ROI). In Fig. 4c, the X-axis is the number of shells included in the ROI, and Y-axis is the AIF averaged over the imaging period. The mean AIF values of the different ROIs were normalized to that of the core shell (Shell 1). Aorta was categorized into 6 groups according to its diameter. In any of the groups, the error in AIF was up to 15%, when a smaller ROI was used.
Conclusions: We developed a semiautomatic technique to determine the AIF in breath-hold DCE-MRI of the abdomen. The error in AIF was significantly reduced by tracking the aorta motion. Also, we confirmed that the semiautomatic segmentation of the aortic region can reduce error in AIF induced by manual segmentation up to 15%.
Acknowledgements
No acknowledgement found.References
1. Kim H, Arnoletti PJ, Christein J, Heslin MJ, Posey JA, 3rd, Pednekar A, Mark Beasley T, Morgan DE. Pancreatic adenocarcinoma: a pilot study of quantitative perfusion and diffusion-weighted breath-hold magnetic resonance imaging. Abdom Imaging 2014; 39:744-752.
2. Kim H, Keene KS, Sarver DB, Lee SK, Beasley TM, Morgan DE, Posey JA, 3rd. Quantitative perfusion- and diffusion-weighted magnetic resonance imaging of gastrointestinal cancers treated with multikinase inhibitors: a pilot study. Gastrointestinal cancer research : GCR 2014; 7:75-81.
3. Tofts PS, Brix G, Buckley DL, Evelhoch JL, Henderson E, Knopp MV, Larsson HB, Lee TY, Mayr NA, Parker GJ, Port RE, Taylor J, Weisskoff RM. Estimating kinetic parameters from dynamic contrast-enhanced T(1)-weighted MRI of a diffusable tracer: standardized quantities and symbols. J Magn Reson Imaging 1999; 10:223-232.
4. Kim H, Samuel S, Totenhagen JW, Warren M, Sellers JC, Buchsbaum DJ. Dynamic contrast enhanced magnetic resonance imaging of an orthotopic pancreatic cancer mouse model. Journal of visualized experiments : JoVE 2015.
5. Otsu N. A threshold selection method from gray-level histograms. IEEE Trans Sys Man Cyber 1979; 9:62-66.
Figures