4836
Fast, Motion-Robust T1-weighted Body Imaging with Single-Shot Fast Spin Echo via Centric Partial Fourier Encoding and Variable Refocusing Flip Angle1Global MR Applications & Workflow, GE Healthcare, New York, NY, United States, 2Global MR Applications & Workflow, GE Healthcare, Menlo Park, CA, United States, 3Global MR Applications & Workflow, GE Healthcare, Houston, TX, United States
Synopsis
Here we present a single-shot fast spin echo sequence optimized for fast, motion-robust T1-weighted body imaging through the use of inversion recovery preparation, and the introduction of variable refocusing flip angle configured for centric, partial Fourier encoding.
Introduction
Single-Shot Fast Spin Echo (SSFSE or HASTE) is revered for its speed, inherent motion robustness, and its excellent T2-weighted contrast. T1-weighted imaging, of interest for routine body imaging, and specialized applications such as whole-body imaging, is typically performed with conventional interleaved multi-shot FSE, which tends to be relatively slow and prone to motion artifacts, in spite of its otherwise exceptional image quality (in terms of signal-to-noise and resolution). For SSFSE, T1-weighting can be introduced with the addition of an inversion recovery preparation pulse (SSFSE-IR) and a centric encoding scheme to minimize the echo time (TE), the main drawback of this approach being the effective doubling of T2-blur due to the interleaving of low-order phase encoding steps1. Here, we attempt to mitigate some of this additional T2-blur for a T1w SSFSE-IR sequence with the introduction of variable refocusing flip angle and partial Fourier to an inversion-recovery prepared SSFSE pulse sequence (vrfSSFSE-IR) with centric encoding.Methods
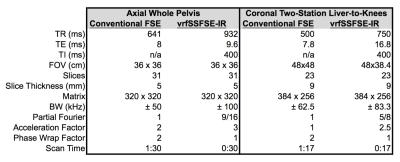
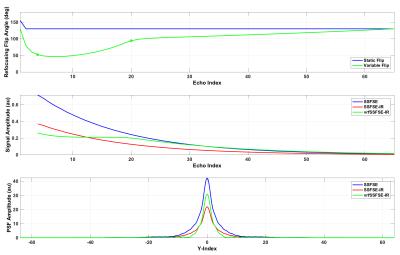
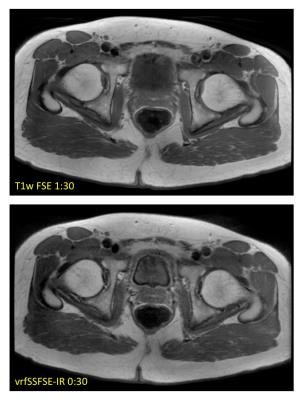
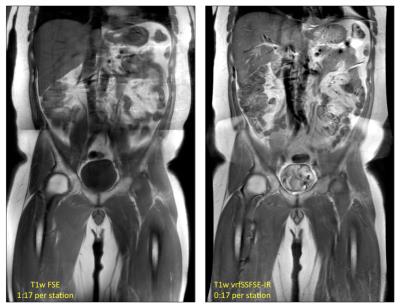
A vrfSSFSE-IR pulse sequence was modified to support centric, partial Fourier encoding and a complimentary refocusing flip angle schedule, characterized by four flip targets: finit 130, fmin 60, fcen 100, and fend 130 degrees2. The centric partial Fourier encoding scheme consisted of two regions, including an early, interleaved segment of low-order phase encoding steps, and a late, linear train of high-order phase encoding steps. The central flip target, fcen, was located at the echo index corresponding to the end of the early, interleaved portion, with the goal of minimizing T2-modulation across this early, interleaved portion of the echo train. Extended phase graph (EPG) simulation results are summarized in Figure 13. Following informed consent, several volunteers were scanned on a 60-cm 3.0T MRI scanner (Discovery MR750, GE Healthcare, Waukesha, WI). For the purposes of demonstration, two body protocols comparing conventional T1w FSE and vrfSSFSE-IR were performed, summarized in Table 1, including an axial acquisition with whole-pelvis coverage, and a coronal 2-station acquisition with liver-to-knees coverage. In the coronal, multi-station acquisition, outer volume suppression in the L/R phase direction was used to eliminate phase wrap from the subject’s arms4.Results
EPG simulation results in Figure 1 for assumed T1/T2 values of 60/1500 ms demonstrate the effects of the IR pulse on the resulting signal amplitude, and the subsequent effects of the variable refocusing flip angles, including a leveling of transverse magnetization across the interleaved portion of the echo train, and a corresponding narrowing of the resulting point spread function in image space. Initial in vivo results are summarized in Figures 2 and 3, showing comparisons of conventional T1-weighted FSE and vrfSSFSE-IR in the axial and coronal scan planes. Scan time for the vrfSSFSE-IR sequence was reduced by a factor of 3 to 5 over conventional FSE, with the exact scan time advantage conferred dependent on slice coverage, acceleration, phase wrap factor and other factors summarized in Table 1.Discussion
These results demonstrate the promise of a fast, motion-robust T1w SSFSE-based pulse sequence. In general, the vrfSSFSE-IR sequence produces image contrast comparable to conventional T1w FSE in substantially less time with fewer motion artifacts, at the modest expense of lower signal-to-noise ratio. T2-mediated blurring is limited with vrf, and effective resolution is increased in the abdomen and other moving anatomical structures for vrfSSFSE-IR due to its inherent ability to freeze motion. In addition, owing to the short TE utilized in T1w imaging, and the short corresponding signal coherence pathway (from excitation to TE), this T1w version of vrfSSFSE-IR is not nearly as susceptible to signal loss and image shading due to cardiac motion as its (long-TE) T2w vrf counterpart, potentially enabling the use of lower minimum refocusing flip angles (fmin) to reduce specific absorption rate and/or to further mitigate T2-blurring5. Further optimization and evaluation of the clinical utility of this technique remains as future work.Conclusion
We have introduced an enhanced version of the SSFSE-IR pulse sequence that utilizes variable refocusing flip angles and centric, partial Fourier encoding to quickly acquire, motion-robust T1-weighted images in the body. We believe this approach may offer valuable workflow advantages without substantial compromises in image quality.Acknowledgements
No acknowledgement found.References
1. Malamateniou C, McGuinness AK, Allsop JM, O'Regan DP, Rutherford MA, Hajnal JV. Snapshot inversion recovery: an optimized single-shot T1-weighted inversion-recovery sequence for improved fetal brain anatomic delineation. Radiology. 2011 Jan;258(1):229-35.
2. Busse RF, Hariharan H, Vu A, Brittain JH. Fast spin echo sequences with very long echo trains: design of variable refocusing flip angle schedules and generation of clinical T2 contrast. Magn Reson Med 2006 May;55(5):1030-7.
3. Hennig, J. Echoes—how to generate, recognize, use or avoid them in MR-imaging sequences. Part II: Echoes in imaging sequences. Concepts Magn. Reson. 1991,3: 179–192.
4. Taviani T, Litwiller D, Loening AM, Manojkumar S, Hargreaves BA, Vasanwala SS. Single-Shot Fast Spin Echo of Targeted Regions with Variable Refocusing Flip Angles and Quadratic Phase Pulses for Outer Volume Suppression. Proceedings of the ISMRM 2015.
5. Litwiller DV, Holmes JH, Saranathan M, Loening AM, Glockner JF, Vasanawala SS, Bayram E. Sensitivity of modulated refocusing flip angle single-shot fast spin echo to impulsive cardiac-like motion. Proceedings of the ISMRM 2014.
Figures