4834
Quantitative Assessment of Markers of Oxidative Stress in Mice Model of Renal Artery Stenosis1Nephrology, Mayo Clinic, Rochester, MN, United States, 2Radiology, Harvard/MGH, Boston, MA, United States, 3Biochemistry and Molecular Biology, Mayo Clinic
Synopsis
Inability of cells to detoxify reactive oxidative species (ROS) is responsible for numerous degenerative pathological conditions. Currently there is no clinical method to assess reduction-oxidation (redox) state in vivo. In this study, using a cyclic nitroxide T1 MR probe with unique characteristics, we propose a two-tissue compartment model which provides quantitative information of markers of oxidative stress. Results demonstrated the feasibility of redox status assessment in vitro and in vivo in the stenotic mouse kidney. In the stenotic kidney, our method indicated increased renal ROS production, accompanied by preserved ability to detoxify ROS compared to the contralateral kidney.
PURPOSE
Unperturbed biological systems have the ability to detoxify reactive oxygen species (ROS) and maintain a delicate reduction-oxidation (redox) balance. Cellular injury may disturb this balance and result in increased oxidative stress, which exerts deleterious effects on proteins, lipids, and DNA, and is responsible for numerous degenerative neurological, cardiovascular, renal, and other pathologies1-3. Currently methods for assessing oxidative stress in vivo are unsuitable for clinical translation, mainly due to their invasive nature (biopsy) or lack of clinical availability (EPR spectroscopy). In this study, we introduce a novel analysis method for quantitative MRI-based assessment of markers of oxidative stress using Tempol, a T1 MR tracer (Figure 1) and superoxide dismutase (SOD) mimetic. The absence of advanced knowledge describing the pharmacokinetics of Tempol within the tissue makes it difficult to discern dynamics of T1 contrast secondary to its reduction or re-oxidization following inflow and clearance from the organ of interest. To alleviate this limitation, we propose a novel kinetic model that accounts for the uptake and washout of Tempol, and deconvolves quantitative markers of ROS generation and cellular detoxification capability from the kinetic information.
METHODS
As a proof-of-concept, using Tempol in an in vitro system, we showed the feasibility of redox state assessment in cells with oxidative stress. The experimental details are discussed in the caption of Figure 2. Imaging was performed on an Avance DRX 700WB (Bruker-BioSpin, Billerica, MA) spectrometer with a vertical 16.4 T wide bore magnet equipped with mini imaging accessories. T1-weighted MR dynamic contrast enhanced (DCE) images with TR/TE = 24/1.68ms, Matrix Size = 128x256, FOV = 2.56x2.56cm with temporal resolution of <3s were acquired over 6-7 minutes. We subsequently investigated this approach in vivo in the stenotic (STK) and contralateral (CLK) kidneys of a mouse model of unilateral renal artery stenosis (RAS) and chronic kidney disease. Five S129 mice underwent unilateral RAS surgery, as described previously4. Blood pressure was measured 3 weeks after RAS induction. Animals were scanned and kidney volumes and perfusion estimated from images collected using respiratory-gated 3D Fast Imaging with Steady Precession (3D-FISP) and arterial spin labelling (ASL), respectively. Tempol DCE imaging was then performed using the same scanning protocol as in cells. Thirty seconds through the imaging, a bolus of 350mM of Tempol was injected through a tail vein catheter over 15 seconds, and acquisition continued for 6-8 minutes (Figure 3). Pharmacokinetic and quantitative oxidative stress markers were assessed by finding the optimal parameters to the differential equations describing our two-tissue compartment model (Figure 4.B) based on our Bayesian estimation method implemented using a Metropolis-Hastings algorithm5,6 and the posterior distribution proposed by Sitek et. al.7 (Figure 4.C).RESULTS
The in vitro experiment showed higher concentration of unreduced Tempol in cells exposed to X/XO compared to untreated myocytes, and indicated that the redox state of cells is detectable with Tempol DCE. In vivo, renal volume and hemodynamics confirmed RAS development in the mice. In a paired statistical analysis, the uptake, 𝑘1, in parallel to perfusion was significantly lower in all STK. While 𝑘3 was not statistically different between STK and CLK (0.46±0.2 vs. 0.69±0.6 min-1, respectively), 𝑘4 tended to be higher in the STK (0.004±0.001 vs. 1.24±0.65 min-1, respectively, p=0.06).DISCUSSION
The in vitro study showed that Tempol DCE-MRI is sufficiently sensitive to detect subtle difference in the concentration of unreduced Tempol in the two samples, despite the significantly lower density of cells in the dialysis bags, and therefore the reduction rate, compared to tissues. The in vivo animal study demonstrated the feasibility of this method for redox state assessment in research and potentially pre-clinical applications. The similarity of 𝑘3 in both STK and CLK showed close agreement to recent studies using histological methods8, which have demonstrated preserved SOD functionality in CKD in the presence of oxidative stress. Together with higher 𝑘4, this observation may suggest that the cellular redox imbalance in the STK is determined by increased ROS production (e.g. as a result of mitochondrial injury) more than by diminished SOD activity.CONCLUSION
Our preliminary results indicate feasibility of assessing renal redox status in vivo using Tempol DCE MRI in RAS. In contrast to previous methods, our approach has the capacity to discrete markers of oxidative stress from wash-in/wash-out driven parameters. Additional studies are needed to test the applicability and sensitivity of this method in a larger group of animals and in additional pathological conditions.Acknowledgements
No acknowledgement found.References
1. Uttara B, Singh AV, Zamboni P, Mahajan RT. Oxidative stress and neurodegenerative diseases: a review of upstream and downstream antioxidant therapeutic options. Current neuropharmacology. 2009;7(1):65-74.
2. Griendling KK, FitzGerald GA. Oxidative stress and cardiovascular injury part I: basic mechanisms and in vivo monitoring of ROS. Circulation. 2003 Oct 21;108(16):1912-6.
3. Stanton RC. Oxidative stress and diabetic kidney disease. Current diabetes reports. 2011;11(4):330-6.
4. Ebrahimi B, Crane JA, Knudsen BE, Macura SI, Grande JP, Lerman LO. Evolution of cardiac and renal impairment detected by high-field cardiovascular magnetic resonance in mice with renal artery stenosis. Journal of Cardiovascular Magnetic Resonance. 2013;15(1):1.
5. Metropolis N, Ulam S. The monte carlo method. Journal of the American statistical association. 1949;44(247):335-41.
6. Metropolis N, Rosenbluth AW, Rosenbluth MN, Teller AH, Teller E. Equation of state calculations by fast computing machines. The journal of chemical physics. 1953;21(6):1087-92.
7. Sitek A, Li Q, El Fakhri G, Alpert NM. Validation of Bayesian analysis of compartmental kinetic models in medical imaging. Physica Medica. 2016;32(10):1252-8.
8. Chang JM, Kuo MC, Kuo HT, Chiu YW, Chen HC. Increased glomerular and extracellular malondialdehyde levels in patients and rats with diabetic nephropathy. Journal of Laboratory and Clinical Medicine. 2005;146(4):210-5.
9. Wilcox CS, Pearlman A. Chemistry and antihypertensive effects of tempol and other nitroxides. Pharmacological reviews. 2008;60(4):418-69.
10. Spinale FG, Mukherjee R, Fulbright BM, Hu J, Crawford FA, Zile MR. Contractile properties of isolated porcine ventricular myocytes. Cardiovascular research. 1993;27(2):304-11.
Figures
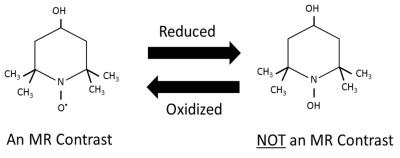
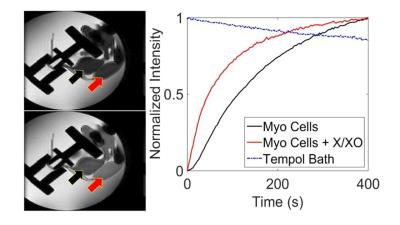
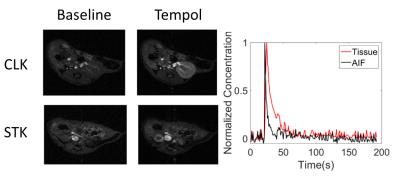
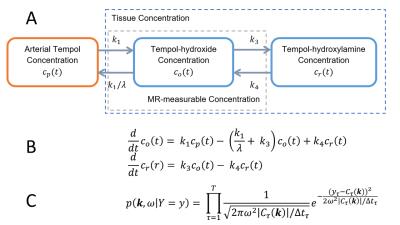
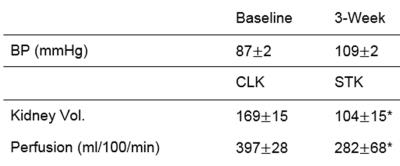
Table 1. Indices of RAS development in mice. Three weeks after RAS, animals tended to be hypertensive (p=0.06); (* p<0.05 vs. CLK).