4832
Quantitative comparison of time-SLIP and Triple Inversion Recovery (TIR) non-contrast enhanced MRI for renal angiographySuzanne Franklin1, Torben Schneider2, Marcelo E Andia3, Markus Henningsson4, and Rene M Botnar4
1Eindhoven University of Technology, Eindhoven, Netherlands, 2Philips Healthcare, Guildford, United Kingdom, 3Pontificia Universidad Católica de Chile, Escuela de Ingeniería, Santiago, Chile, 4King’s College London, Division of Imaging Sciences, and Biomedical Engineering, St. Thomas' Hospital, London, United Kingdom
Synopsis
Renal artery stenosis (RAS) has been associated with hypertension, chronic kidney disease and an increased risk of vascular events. This study quantitatively compares two non-contrast enhanced magnetic resonance angiography (NC-MRA) techniques for renal angiography, based on SNR, CNR, vessel sharpness and number of renal branches. TIR has shown promising results in suppressing background signal and provided overall better image quality than outflow time-SLIP. TIR has the advantage over time-SLIP that tissues can be nulled over a wide range of T1 -values. To further shorten scan time the TIR technique could be combined with more advanced image based motion correction techniques.
Introduction
Renal artery stenosis (RAS) has been associated with hypertension, chronic kidney disease and an increased risk of vascular events1. RAS can be reliably diagnosed using contrast-enhanced magnetic resonance angiography (CE-MRA). However, CE-MRA cannot be used in patients with renal dysfunction, since gadolinium-based contrast agents can cause nephrogenic systemic fibrosis2. This has sparked the interest for non-contrast enhanced magnetic resonance angiography (NC-MRA).Methods
In this study we investigate two NC-MRA techniques, outflow time-spatial labeling inversion pulse (time-SLIP)3 and Triple Inversion Recovery (TIR)4. Outflow time-SLIP consists of one non-selective inversion pulse for background suppression and one spatially selective inversion pulse for arterial blood labeling. In contrast, the TIR technique consists of two non-selective and one spatially selective inversion pulse as shown on figure 1a and b. The two non-selective inversion pulses enable the TIR technique to null tissue over a wide range of T1-values while time-SLIP only allows nulling the signal of one T1 species. The inversion times (TI1 and TI2) of the TIR sequence were optimized, as described by Andia et al. (2012)4 to provide maximal contrast between background and blood signal as shown in figure 1c. The selective inversion pulse of TIR allows labeling the inflowing blood in the thoracic aorta. The choice of the inversion time for time-SLIP (TI, see fig 1a) is a trade-off between background suppression and the distance blood can travel to reach the distal renal branches. A TI of 400ms was empirically found to be optimal, see figure 2. Seven healthy adults, four male and three female, were scanned on a Philips 1.5T Ingenia MR scanner (Philips Healthcare, Best, The Netherlands) using 32ch-anterior and integrated 28ch-posterior coils. In each subject both NC-MRA techniques were performed using a 3D balanced SSFP acquisition to achieve high signal an contrast to noise ratio (SNR, CNR). Imaging parameters included TR/TE of 6.6ms/3.3ms, SENSE factor of 2, FOV 347x347, voxel size 1.5x1.55x1.5, matrix 232x221, and ten start-up echoes with linear ramp-up. This led to a scan time of 1:33min for both techniques. For respiratory motion compensation, a diaphragmatic navigator was used with a gating window of 5mm. In case of time-SLIP a SPIR pulse was used to suppress the fat signal. A SPIR pulse was not necessary for the TIR sequence, since the fat signal is suppressed by the nonselective inversion pulses. A separate noise scan was performed to establish the noise level for SNR and CNR calculation. The two techniques were quantitatively compared based on SNRblood, CNRkidney, CNRliver, vessel sharpness and number of visible renal branches.Results
TIR renal angiography resulted in superior background suppression compared to outflow time-SLIP as shown in figure 3. Visual findings are in good agreement with improved SNR, CNR, vessel sharpness and number of renal branch vessels visualized (figure 4). The number of visible renal branches is an important clinical measure for RAS diagnosis. Difference in quantitative parameters between the two methods were tested for significance with a nonparametric Wilcoxon test, but none was found to be significant.Discussion and conclusion
In this study TIR has shown promising results in suppressing background signal and provided overall better image quality than outflow time-SLIP. TIR has the advantage over time-SLIP that tissues can be nulled over a wide range of T1 -values. Improved background suppression leads to higher vessel sharpness and combined with a longer inflow time results in a superior visualization of the renal branches compared to outflow time-SLIP, which is important for RAS diagnosis. A potential limitation of TIR is that it is more suitable for relatively low heart rates, because of the long inversion times. For higher heart rates, more than one cardiac beat could be used to accommodate longer inversion times at the expense of increased scan time. To further shorten scan time the TIR technique could be combined with more advanced image based motion correction techniques incorporating also non-rigid motion correction that would allow for 100% scan efficiency.Acknowledgements
This work was supported by the British Heart Foundation (BHF, RG/12/1/29262) and Philips Healthcare.References
1. Lance D. Dworkin and Christopher J. Cooper. Renal-Artery Stenosis. New England Journal of Medicine, 361(20):1972–1978, 2009. 2. Henrik S. Thomsen. Gadolinium-based contrast media may be nephrotoxic even at approved doses. European Radiology, 14(9):1654–1656, 2004. 3. Mitsue Miyazaki and Masaaki Akahane. Non-contrast enhanced MR angiography: Established techniques. Journal of Magnetic Resonance Imaging, 35(1):1–19, 2012. 4. Marcelo E. Andia and Rene M. Botnar. Arterial spin labeling angiography using a triple inversion recovery prepulse. Magnetic resonance in medicine, 67(2):477–83, 2012.Figures
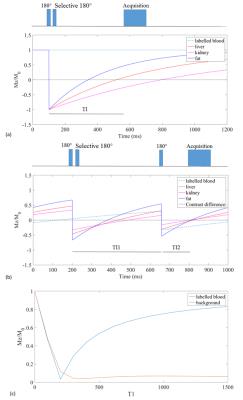
Figure 1: Pulse sequences for time-SLIP and
TIR spin labeling angiography. (a) Time-SLIP sequence. A non-selective inversion pulse, followed by selective reinversion of the labeling
slab, restoring the blood signal. Data acquisition coincides with the zero-crossing
of the background magnetization.
(b) TIR sequence. A non-selective inversion
pulse, followed by selective reinversion of the labeling slab, restoring the
blood signal. After TI1 another inversion pulse is applied, inverting both
background and blood signal. (c) Optimisation of TI1 and TI2 for TIR sequence to
maximize the difference between labelled blood and background signal at the time
of acquisition.

Figure 2: Maximum
intensity plots of time-SLIP comparing inversion times (TI) of TI=400ms
(a) and TI =560ms (b). (a) Volunteer C, time-SLIP with TI=400ms, as
used in the study resulted in SNRblood,
CNRkidney , CNRliver, and vessel sharpness values
of respectively 90/75/63/0.56. (b) Volunteer
C, time-SLIP with TI=560ms. This gave the
blood more time to reach the distal branches, but resulted in suboptimal
background suppression and consequently led to lower values on SNRblood,
CNRkidney , CNRliver, and vessel sharpness, respectively
50 /31/21/0.45.
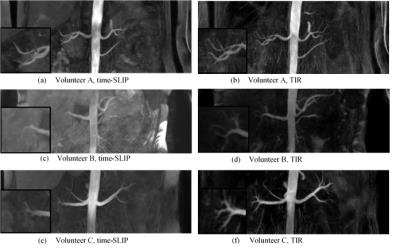
Figure
3: Maximum intensity plots of time-SLIP
and TIR spin labeling
angiography of renal arteries in data for three volunteers.
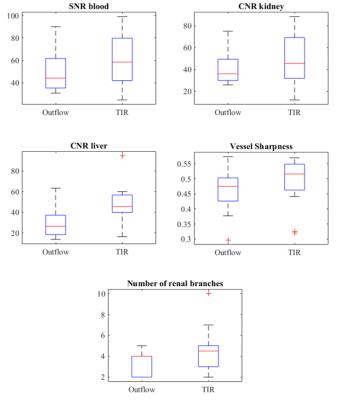
Figure
4:
Boxplots
representing the results of the measurement
parameters comparing time-SLIP and TIR techniques.