4830
SAR-Constrained kT-Points Pulse Design Applied to B1 Inhomogeneity Mitigation in the Human Abdomen at 3T1CEA-DRF-I2BM-NeuroSpin-UNIRS, Gif-sur-Yvette, France, 2Siemens Healthineers France, Saint-Denis, France, 3Department of Radiology, AP-HP, CHU Henri Mondor, Créteil, France
Synopsis
High field MRI systems offer better performance in terms of signal-to-noise ratio but are burdened with dielectric resonance artefacts inducing zones of weak excitation with major consequences on Signal and Contrast to Noise Ratios. In this work, the interest of subject-tailored kT-points pulse design with joint SAR control over current patient-specific RF-shimming technique is investigated, in the context of human liver imaging at 3T. T1w acquisitions are performed in-vivo to compare quadrature, tailored RF-shimming, and kT-points pulses. The interest of kT-points is clearly demonstrated in terms of signal, contrast and diagnostic power.
Introduction
3T MRI systems are now widely available and extensively used in clinical environments as they provide a better signal-to-noise ratio (SNR), which can be used to improve spatial or temporal resolution.1 However, the dielectric resonance artefacts induced in regions whose size is close to the radiofrequency (RF) wavelength lead to loss of signal and contrast in some areas of larger organs such as the liver.1-3 Since the advent of two-channel parallel transmission (pTX) clinical scanners, subject-specific static RF shimming has been admitted as an efficient solution to this issue. 4,5 Dynamic pTX approaches 3,6 have been introduced, following the example of ultra-high field MRI, but without explicit nor look-ahead SAR control. The kT-points approach, however, has demonstrated excellent flip angle (FA) homogenisation performance for non-selective excitation in the brain at 7T, 7,8 while ensuring a safe SAR level. In this work, the applicability of kT-points to achieve excitation homogeneity over a human abdomen in a clinical environment on a CE-labelled 3T scanner is analysed. To the authors’ knowledge, this is the first evaluation of dynamic excitation homogenisation in the abdomen at 3T under strict SAR constraint.Materials and Methods
Acquisitions were carried out on a clinical Siemens MAGNETOM Skyra 3T scanner (Siemens Healthineers, Erlangen, Germany), fitted with a product two-channel pTX system. Measurements were performed on the abdomen of a 38-year-old female patient (BMI = 30) investigated for characterisation of a hepatic nodule. In order to rely as much as possible on tools available on product scanners, B1+ maps were obtained through a manufacturer standard adjustment procedure, along with the associated virtual observation points 9 (VOP) used for SAR calculation. Although an f0 map – needed for the pulse design – was also provided to inform of local variations in resonance frequency with respect to the main field (B0 inhomogeneity, chemical shift), it was subject to breathing artefacts and its spectral resolution was insufficient for fatty tissues. A custom-made breath-hold f0 sequence was therefore inserted instead. Three pulses were then compared: standard circularly polarised (CP) mode (“TrueForm C”), manufacturer subject-tailored RF-shimming (so called “Volume-selective“), and kT-points. A 7° flip angle was targeted and each pulse was incorporated in a “dynamic enhancement” 3D GRE T1-weighted-like sequence performed in a single breath-hold, before contrast agent injection. Acquisition parameters were: 320x220x72 matrix, 1.1x1.1x3mm3 resolution, TE/TR= 3/6ms, iPAT factor 2, 80%/50% phase/slice resolution, TA= 23s). The 7° 7-kT-point pulse was designed using MATLAB’s (The Mathworks, Natick, MA, USA) built-in active-set algorithm; flip angles were evaluated by numerical Bloch integration. The number of kT-points was chosen to be seven to allow as many degrees of freedom as possible while maintaining a reasonably short pulse duration. Flip angle NRMSE (normalised root-mean-square error from the target over the volume of interest) was minimised by optimising simultaneously RF complex coefficients, kT-points locations and durations under SAR and hardware constraints.10,11 Taking advantage of general-purpose GPU computing allowed to design the pulse in less than one minute.Results
Axial views of the T1w acquisitions are shown in Figure 1: results were compared between the three transmit modes. Pre-acquisition normalisation was performed to eliminate reception profiles as much as possible. The characteristic shading due to dielectric resonance has almost disappeared on the kT-points images, while it was still present with tailored RF-shimming. Anatomical structures inside and near the liver, such as the aorta, the portal system and the gallbladder, are also much better resolved. These results are confirmed in Figure 2, where one can see that kT-points achieve homogenisation not only in the presented slices, but in the whole volume. For instance, artefacts are greatly reduced in the left lobe of the liver below the heart, as well as in the hepatic dome. Characteristics of the three pulses used are gathered in Table 1.Conclusion
Excitation homogenisation in the abdomen is necessary to ensure reliable diagnostic in the liver at 3T. These preliminary results highlight the fact that tailored static RF-shimming is not satisfactory enough to justify the use of two-channel pTX. The kT-points technique, on the other hand, fully embraces pTX capabilities and allows better SNR and contrast recovery than RF-shimming, thus an increased diagnostic power in the liver. This is achieved with a controlled SAR, and requires less than one minute of computation time. A study is currently being done to analyse the robustness of the approach. In the near future, we plan to extend this method to slab-selective excitation and fat-saturation pulses.Acknowledgements
The authors wish to thank all the MRI technicians of Henri Mondor Hospital for their patience, understanding and helpful clinical advices. This project was funded by CEA’s Programme Transversal, Technologies pour la Santé (Transversal Programme for Health Technologies).References
1. Merkle, E.M., Dale, B.M., Paulson, E.K., 2006. Abdominal MR Imaging at 3T. Magnetic Resonance Imaging Clinics of North America 14, 17–26. doi:10.1016/j.mric.2005.12.001
2. Bernstein, M.A., Huston, J., Ward, H.A., 2006. Imaging artifacts at 3.0T. J. Magn. Reson. Imaging 24, 735–746. doi:10.1002/jmri.20698
3. Padormo, F., Beqiri, A., Hajnal, J.V., Malik, S.J., 2015. Parallel transmission for ultrahigh-field imaging: Parallel Transmission for Ultrahigh-Field Imaging. NMR in Biomedicine n/a-n/a. doi:10.1002/nbm.3313
4. Kukuk, G.M., Gieseke, J., Nelles, M., König, R., Andersson, M., Muschler, E., Mürtz, P., Stout, J., Nijenhuis, M., Träber, F., others, 2009. Clinical liver MRI at 3.0 Tesla using parallel RF transmission with patient-adaptive B1 shimming, in: Proc. Intl. Soc. Mag. Reson. Med. p. 119.
5. Childs, A.S., Malik, S.J., O’Regan, D.P., Hajnal, J.V., 2013. Impact of number of channels on RF shimming at 3T. MAGMA 26, 401–410. doi:10.1007/s10334-012-0360-5
6. Malik, S.J., Keihaninejad, S., Hammers, A., Hajnal, J.V., 2012. Tailored excitation in 3D with spiral nonselective (SPINS) RF pulses. Magn. Reson. Med. 67, 1303–1315. doi:10.1002/mrm.23118
7. Amadon, Alexis, Cloos, M.A., 2011. Method and apparatus for compensating for B1 inhomogeneity in magnetic resonance imaging by nonselective tailored RF pulses. WO2011/128847A1.
8. Cloos, M.A., Boulant, N., Luong, M., Ferrand, G., Giacomini, E., Le Bihan, D., Amadon, A., 2012. kT-points: Short three-dimensional tailored RF pulses for flip-angle homogenization over an extended volume. Magnetic Resonance in Medicine 67, 72–80. doi:10.1002/mrm.22978
9. Eichfelder, G., Gebhardt, M., 2011. Local specific absorption rate control for parallel transmission by virtual observation points. Magn. Reson. Med. 66, 1468–1476. doi:10.1002/mrm.22927
10. Tomi-Tricot, R., Gras, V., Boulant, N., Vignaud, A., Amadon, A., 2016. kT-Points Pulse Design at 7T: Optimization of Pulse and Sub-Pulse Durations. 33rd Annual Scientific Meeting, Vienna, AT: Abstracts. Magnetic Resonance Materials in Physics, Biology and Medicine 29, 247–400. doi:10.1007/s10334-016-0570-3
11. Hoyos-Idrobo, A., Weiss, P., Massire, A., Amadon, A., Boulant, N., 2014. On variant strategies to solve the magnitude least squares optimization problem in parallel transmission pulse design and under strict SAR and power constraints. Medical Imaging, IEEE Transactions on 33, 739–748.
Figures
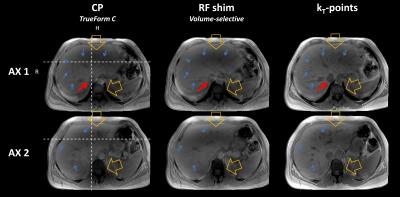
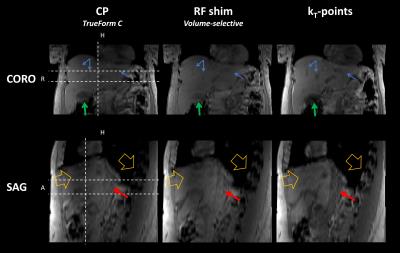
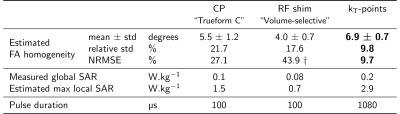
Table 1. Homogenisation performance, time and safety characteristics of the three pulses analysed. Estimated local SAR was calculated from the VOPs provided by the scanner. Estimations of flip angle mean, standard deviation and NRMSE were obtained by numerical Bloch simulations based on f0 and B1+ maps from the calibration step. The kT-points pulse, designed under explicit SAR, hardware and time constraints, achieves better homogeneity than CP and tailored RF-shim, at the cost of a slightly higher SAR and a longer duration. $$$\dagger$$$ NRMSE may not be relevant for RF-shim as it might not be the criterion chosen by the manufacturer.