4820
Accelerated HASTE-Based Fetal MRI with Low-Rank Modeling1Martinos Center for Biomedical Imaging, Chalestown, MA, United States, 2Department of Radiology, Harvard Medical School, Boston, MA, United States, 3Boston Children's Hospital, Boston, MA, United States, 4Department of Electrical Engineering and Computer Science, Massachusetts Institute of Technology, Cambridge, MA, United States
Synopsis
HAlf-fourier Single-shot Turbo spin Echo (HASTE) sequence is one of the most common acquisitions in fetal MRI due to its T2 contrast and relative robustness to fetal motion. In this work, we present a low-rank model-based imaging method to accelerate HASTE acquisitions. The proposed method is fully compatible with the k-space sampling strategy implemented by vendor-provided pulse sequences, and provides improved image quality and/or noise robustness compared to the conventional half-Fourier and GRAPPA reconstruction, and compressive sensing reconstruction.
Introduction
HAlf-fourier Single-shot Turbo spin Echo (HASTE) imaging has been a major workhorse for fetal MRI due to its T2 contrast and relative immunity to fetal motion [1]. HASTE acquisitions collect data from only more than half of the k-space, in which the central k-space are fully-sampled while structure subsampling is performed in the outer k-space. The conventional reconstruction approach first utilizes the GRAPPA reconstruction [2] to fill in missing k-space data in the sub-sampled region, followed by the half-Fourier reconstruction [3] to obtain the remaining half of the k-space data. While this approach provides good performance for clinical applications at low undersampling levels, its performance can dramatically degrade due to severe SNR penalty as the undersampling level increases. Compressive sensing (CS) [4, 8] is a more recent approach that enables image reconstruction from undersampled data. However, CS reconstruction can result in significant blurring at a higher acceleration factor, reducing its diagnostic utility. Moreover, for best performance, CS reconstruction generally relies on random sampling strategies. In this abstract, we present a low-rank model-based imaging method to accelerate HASTE acquisitions for fetal MRI. The proposed method accommodates the existing sampling strategy in the vendor-provided pulse sequences, and provides improved image quality and noise robustness compared to the conventional approach and CS reconstruction. Representative results from in vivo experiments are shown to illustrate the performance of the proposed method.Method
It has been recently demonstrated in [5, 6] that the linear predictability of local k-space neighborhood, associated with a support-limited image function, can be used to form an approximately low-rank matrix. Further, taking into account the inter-channel correlations in parallel imaging [7], we can construct a joint matrix $$$\mathbf{C}=[\mathbf{C}_1,\cdots,\mathbf{C}_L]$$$ with an even more parsimonious low-rank representation. With this observation, image reconstruction problem can be formulated as a low-rank matrix recovery problem. Assuming that $$$\rho(\mathbf{k})$$$ denotes the complete set of k-space data, the image reconstruction problem can be written as
$$\hat \rho \left( {\bf{k}} \right) = \arg \mathop {\min }\limits_{\rho \left( {\bf{k}} \right)} \left\| {\Omega (\rho \left( {\bf{k}} \right)) - {\bf{d}}} \right\|_2^2 + \lambda {\Psi _r}({{\cal P}_c}\left( {\rho \left( {\bf{k}} \right)} \right)),$$
where $$$\Omega(\cdot)$$$ denotes the sparse sampling operator, $$$\mathbf{d}$$$ the measured k-space data, $$$\Psi_r(\cdot)$$$ a rank-promoting regularization functional (e.g., the nuclear norm or Shattern p-norm), $$$\mathcal{P}_c(\cdot)$$$ the linear operator that embeds local k-space neighborhoods into a low-rank matrix, and $$$\lambda$$$ the regularization parameter. Note that a number of numerical algorithms can be applied to solve the above optimization problem, e.g., majorization-minimization algorithm [6]. After determining $$$\rho(\mathbf{k})$$$, the corresponding image function can be readily reconstructed.
Results
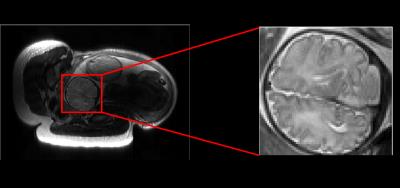
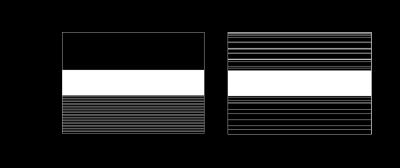
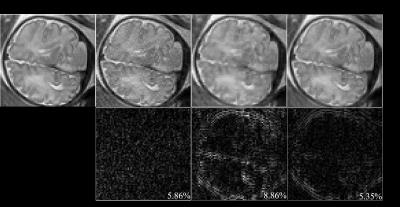
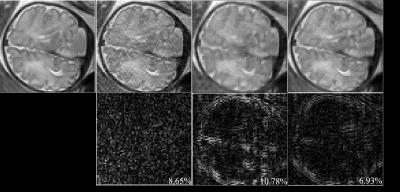
Here, we performed a feasibility study of the proposed method with in vivo fetal imaging data. The experiment was conducted on a 3T Siemens Skyra scanner using the HASTE sequence. The relevant imaging parameters include: FOV = 360×360 mm2, slice thickness = 3.5 mm, matrix size = 256×512, and flip angle = 160º. We fully sampled 5/8 of the entire k-space, and performed the half-Fourier reconstruction to obtain a reference data set (as shown in Figure 1). We then performed a retrospective undersampling using the sampling schemes shown in Figure 2, and reconstructed images using three different methods: (1) conventional approach (i.e., Half-Fourier + GRAPPA), (2) CS reconstruction, and (3) the proposed low-rank reconstruction. More specifically, for the conventional approach and the proposed method, we used the k-space undersampling strategy implemented by the vendor-provided HASTE sequence. For CS, we performed random sampling for the outer k-space region, while keeping the central k-space fully sampled as in the other two approaches. Here, undersampling was performed at two levels, resulting in two net acceleration factors, i.e., 2.67 and 2.91. Figure 3 and 4 respectively show the corresponding reconstruction results (zoomed-in to the fetal brain). As can be seen, the proposed method significantly outperforms the conventional approach and CS reconstruction at a higher acceleration factor. In particular, compared to the conventional approach, the proposed method effectively alleviates the SNR penalty with the use of the low-rank model. Compared to the CS reconstruction, the proposed method largely overcomes the blurring artifacts, which is highly desirable.Conclusion
This abstract presented
a low-rank model-based reconstruction method to accelerate HASTE imaging for
fetal MRI. The proposed method is fully compatible with the k-space undersampling
trajectories in the vendor-provided pulse sequences, and yields improved image
quality and/or SNR compared to the conventional approach and compressive
sensing reconstruction.Acknowledgements
This work was supported in part by the following research grants: NIH-R01EB017337 and NIH-U01-MH093765.
References
[1] A. Gholipour, J. A. Estroff, C. E. Barnewolt, R. L. Robertson, P. E. Grant, B. Gagoski, S. K. Warfield, O. Afacan, S. A. Connolly, J. J. Neil, A. Wolfberg, and R. V. Mulkern. “Fetal MRI: A technical update with educational aspirations,” Concepts Magn. Reson. Part A, vol. 43, pp. 237-266, 2014.
[2] M. A. Griswold, P. M. Jakob, R. M. Heidemann, M. Nittka, V. Jellus, J. Wang, B. Kiefer, and A. Haase. “Generalized autocalibrating partially parallel acquisitions (GRAPPA),” Magn. Reson. Med., vol. 47, pp. 1202-1210, 2002.
[3] D. C. Noll, D. G. Nishmura, and A. Macovski, “Homodyne detection in magnetic resonance imaging,” IEEE Trans. Med. Imag. vol. 10, pp. 154-163, 1991.
[4] M. Lustig, D. Donoho, and J. M. Pauly, “Sparse MRI: The application of compressed sensing for rapid MR imaging,” Magn. Reson. Med., vol. 58, pp. 1182-1195, 2007.
[5] P. J. Shin, P. Larson, M. A. Ohliger, M. Elad, J. M. Pauly, D. B. Vigneron, and M. Lustig. “Calibrationless parallel imaging reconstruction based on structured low-rank matrix completion,” Magn. Reson. Med. vol. 72, pp. 959–970, 2014.
[6] J. P. Haldar, “Low-rank modeling of local k-space neighborhoods (LORAKS),” IEEE Trans. Med. Imag., vol. 33, pp. 668-681, 2014.
[7] J. P. Haldar, and J. Zhuo, “P-LORAKS: Low-rank modeling of local k-space neighborhoods with parallel imaging data.” Magn. Reson. Med. vol. 75, pp. 1499–1514, 2016.
[8] D. Liang, B. Liu, J. Wang, L. Ying, “Accelerating SENSE using compressed sensing,” Magn. Reson. Med. vol. 62, pp. 1574-1584, 2009.
Figures