4815
Preliminary Experience Using Motion-Robust Dynamic MRI to Visualize Fetal Congenital Heart Disease: Comparison to Static MRI1Medical Biophysics, University of Toronto, Toronto, ON, Canada, 2Physiology and Experimental Medicine, Hospital for Sick Children, Toronto, ON, Canada, 3Pediatric Cardiology, Hospital for Sick Children, ON, Canada, 4Pediatric and Diagnostic Imaging, University of Toronto, Toronto, ON, Canada
Synopsis
Recent advances in cardiac MRI have enabled powerful new methods for assessing the fetal heart in utero. Using a novel reconstruction framework, combining methods for motion correction, retrospective gating, and accelerated imaging, motion-robust CINE images are reconstructed and compared to conventional static MRI of the fetal heart. Preliminary evaluation of fetal congenital heart disease with this technique is demonstrated in multi-slice axial acquisitions of four subjects.
Introduction
In pregnancies where fetal cardiovascular abnormalities are present, in utero imaging has become an integral facet of patient care. While ultrasound is the primary modality for fetal cardiac imaging, there is growing interest in using MRI to study the fetal heart (1,2). Studies using static MRI are possible but may be affected by artifact from maternal respiration, fetal cardiac motion, and gross fetal movement (3). Recent advances to overcome these limitations and obtain dynamic images of the fetal heart include retrospective cardiac gating (4,5), accelerated imaging (6), and motion correction (7). Here we present our preliminary experience using such methods to visualize fetal congenital heart disease. The image quality of this novel reconstruction approach was scored, relative to static MRI, by an expert reviewer.Methods
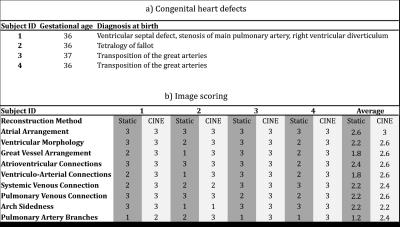
Continuous golden angle radial MRI data were acquired in five human fetuses. For each volunteer, ten axial slices were prescribed spanning the heart on a Siemens 1.5T clinical system using both body and spine matrices (Avanto Fit, Siemens Healthcare – Germany). One subject was excluded from further analysis due to poor slice prescription. Scans were performed free-breathing with the following acquisition parameters: flip angle: 70°, acquired spokes: 3000, TR: 4.95 ms, samples per spoke: 256, field-of-view: 256 x 256 mm2, spatial resolution: 1 x 1 x 4 mm3, and scan length: ~15 s per slice. Each acquisition was reconstructed in two ways. First, static images were reconstructed using only the first 400 spokes (Nyquist sampling criterion). These reconstructions represented the conventional method for fetal cardiac MRI (3). Second, CINE reconstructions of the entire 3000 spokes were performed using a novel imaging approach wherein an initial real-time reconstruction of the data was used to visually assess and reject data undergoing through-plane motion (5), calculate in-plane motion using rigid registration (7), and extract a cardiac gating signal (4). The original data were then motion corrected, sorted by cardiac phase and reconstructed using compressed sensing to produce motion-robust CINE images (6,8). For each subject, a congenital heart defect was identified by ultrasound prior to the MRI examination and confirmed at birth. The gestational ages at the time of the MRI and the post-natal diagnoses are listed in Table 1a. Static and CINE images were presented as DICOMS and analyzed by an expert reviewer (MS). To assess the quality of static and CINE images, each method was evaluated based on a three-point scoring system of the nine cardiac structures listed in Table 1b. Three points were awarded for clear identification of anatomical structures, two were awarded for identifiable structures obscured by artifact and one point was awarded for non-identifiable structures. For each case, the static images were scored first and then the CINE images were scored for the same patient. The added value provided by CINE imaging was then determined by comparing the image quality scores for each case and the difference between the two methods was evaluated using a Wilcoxon test.Results
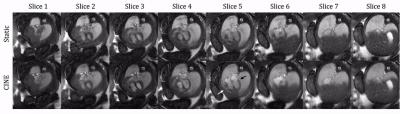
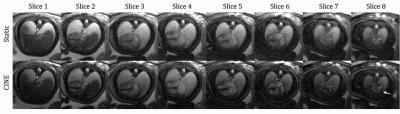
Table 1b displays image scores for both static and CINE images of the fetal heart. Overall, the CINE images scored significantly higher using a Wilcoxon test (p = 10-4). Similarly, the average score for individual structures was consistently higher for CINEs except for arch sidedness which was equally assessed by static images. Figs. 2-5 show both static and animated CINE reconstructions (click on figures to play videos) of representative slices from the four subjects. Overall, image quality for CINE reconstructions is noticeably better than for static images, echoing the tabulated scores. For subject 1 (Fig. 2), most cardiac structures could be identified from the static images, due to the minor motion observed in real-time reconstructions of this acquisition. However, quickly moving dynamic structures such as the atrioventricular valves (example denoted by the black arrow in Fig. 2 slice 5) and a right ventricle diverticulum (white arrow in Fig. 2 slice 5) are better visualized by the CINE images. Conversely, for subject 2 (Fig. 3) the presence of more dramatic motion resulted in noticeable blurring of the great vessels (Fig. 3 slice 7 & 8), which impeded identification of their arrangement. Both Figs. 4 and 5 show further examples of abnormal configurations of the great vessels and once again the motion-robust CINE reconstructions provide improved visualization of dynamic cardiac anatomy.Discussion
Using this novel approach to motion-robust CINE reconstruction, fetal congenital heart defects were evaluated in utero with improved visualization of cardiac structures relative to static MRI. These preliminary results motivate studies in a larger fetal population and comparison to ultrasound to determine the value of this new approach.Acknowledgements
No acknowledgement found.References
1. Godfrey ME., Messing B., Cohen SM., Valsky D V., Yagel S. Functional assessment of the fetal heart: a review. Ultrasound Obstet Gynecol 2012;39(2):131–44. Doi: 10.1002/uog.9064.
2. Pugash D., Brugger PC., Bettelheim D., Prayer D. Prenatal ultrasound and fetal MRI: The comparative value of each modality in prenatal diagnosis. Eur J Radiol 2008;68(2):214–26. Doi: 10.1016/j.ejrad.2008.06.031.
3. Dong S-Z., Zhu M., Li F. Preliminary experience with cardiovascular magnetic resonance in evaluation of fetal cardiovascular anomalies. J Cardiovasc Magn Reson 2013;15(1):40. Doi: 10.1186/1532-429X-15-40.
4. Roy CW., Seed M., van Amerom JFP., et al. Dynamic imaging of the fetal heart using metric optimized gating. Magn Reson Med 2013;70(6):1598–607. Doi: 10.1002/mrm.24614.
5. Chaptinel J., Mivelaz Y., Yerly J., et al. A Golden-Angle Acquisition Coupled with k-t Sparse SENSE Reconstruction for Fetal Self Retro-Gated Cine Cardiac MRI: an In Vivo Feasibility Study. Int Soc Magn Reson Med 2016;(3):459.
6. Roy CW., Seed M., Macgowan CK. Accelerated MRI of the fetal heart using compressed sensing and metric optimized gating. Magn Reson Med 2016;0:1–11. Doi: 10.1002/mrm.26290.
7. van Amerom JFP., Kuklisova Murgasova M., Price AN., et al. Fetal cardiac cine imaging from motion-corrected super-resolution reconstruction of highly-accelerated real-time MRI. Proc Int Soc Magn Reson Med 2016:458.
8. Roy CW., Seed M., Macgowan CK. High resolution multislice imaging of the fetal heart using MOG and iGRASP. Scmr 2016;18(Suppl 1):1–2. Doi: 10.1186/1532-429X-18-S1-P44.
Figures