4809
Characterization of the placental vascular tree using MRI: an ex-vivo study1Sackler Faculty of Medicine, Tel Aviv University, Tel Aviv, Israel, 2Functional Brain Center, The Wohl Institute for Advanced Imaging, Tel Aviv Sourasky Medical Center, Tel Aviv, Israel, 3Department of Obstetrics and Gynecology, Lis Maternity Hospital, Tel Aviv Sourasky Medical Center, Tel Aviv, Israel, 4Division of Pediatric Radiology, Tel Aviv Sourasky Medical Center, Tel Aviv, Israel, 5Pediatric Neurology, Tel Aviv Sourasky Medical Center, Tel Aviv, Israel, 6Sagol School of Neuroscience, Tel Aviv University, Tel Aviv, Israel
Synopsis
Placental vascular dysfunction is a major cause of pregnancy complications, such as intrauterine growth restriction (IUGR). However, current knowledge on human placental vascular architecture is limited. In this study, for the first time, we characterized the structure of the placental vascular system ex-vivo, using MRI. Fifteen normal placentas and one with IUGR were studied using a novel method, and the vascular structure was analyzed with an automatic algorithm. Results provided information regarding: cord insertion location; branching pattern; branching generation; and daughter to mother diameters of normal placentas. Preliminary results from one IUGR placenta suggest significant differences from normal placentas.
Purpose
The placenta is an essential organ for normal development of the fetus. Placental dysfunction is a major cause of pregnancy complications, such as intrauterine growth restriction (IUGR). Examination of placental vascular tree perfusion and structure is important for assessing placental pathologies, yet, human placental vascular tree architecture is poorly understood. The aim of this work was to study and characterize the structure of the placental vascular system ex-vivo using high-resolution MRI, by developing a method for automatic vasculature characterization.Methods
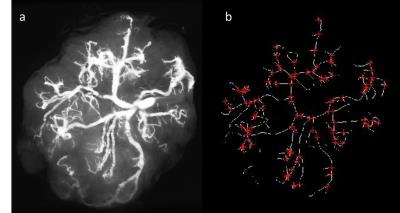
Sixteen placentas, fifteen normal and one with IUGR were obtained from donors who signed an informed consent. Normal placentas were taken from full term pregnancies (38-41 weeks’ gestation) up to two hours after delivery of healthy newborns weighing between 2700 kg to 3900 kg. An IUGR placenta was taken immediately after cesarean section of a preterm (30 weeks’ gestation) baby weighing 730 gr. Placentas were rinsed with a solution of saline and heparin and then a solution of gelatin and contrast agent was injected into the umbilical vessels based on Rasmussen et al1. Structural ex-vivo MR scan of the placenta using high resolution T1 weighted images (0.2x0.2x0.4mm) was performed (see Figure 1a). An automatic placental vascular tree segmentation and characterization method was developed consisting of the following steps: (1) Vascular tree segmentation by threshold; (2) Curve-skeleton and vascular branching definition using specific modules in VSG image processing and analysis toolbox version 3.22 (see Figure 1b); (3) Diameter calculation of any defined blood vessel segment by finding the middle column of the segment after rotating it horizontally by the angle calculated by Inverse Tangent. The accuracy of the method was tested against manual bifurcation count in all 15 placentas and manual diameter measurements (pixel counting) of 2 representative vascular segments in 5 placentas (total of 10 different blood vessels). Placental vascular architecture was studied in accordance to a previous ex-vivo vascular corrosion casting study3, including: (a) cord insertion location: central vs. marginal; (b) branching pattern identification: dichotomous vs. monopodial; Dichotomous pattern defines a symmetric network in which each vessel branches into two fairly similar daughter vessels; monopodial pattern defines a network in which a main vessel courses for a long distance with small diameter tubes branching off to the sides; (c) branching generation; and (d) daughter (vessel branching from a main vessel) to mother (the main vessel of the last generation) diameters.Results
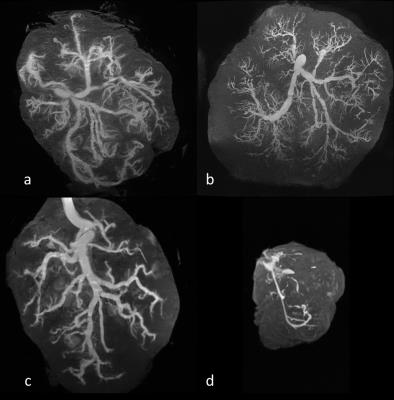
Fig. 2 shows representative MRI scans of normal placentas with central cord insertion (a,b), a normal placenta with marginal cord insertion (c) and a placenta with IUGR (d), using high resolution T1 weighted images after gelatin and contrast agent injection into the umbilical vessels. The automatic placental vascular tree segmentation and characterization method was successfully applied in all placentas. A high correlation was obtained between the algorithm and the manual results both for vessel diameter (r=0.99) and for number of bifurcations (r=0.96). Observation of placental vascular architecture revealed 4 placentas with marginal cord insertion vs. 11 with central cord insertion. Branching pattern was detected up to the 6th generation, and revealed a higher number of monopodial bifurcation pattern in cases of marginal cord insertion. Daughter-to-mother vessel diameter ratios showed a mean of 0.59 ± 0.044 for a dichotomous branching and a mean of 0.18 ± 0.015 for a monopodial branching. Diameters of the first dichotomous arterial branches ranged from 1.2 to 6 mm. These results are in line with other studies of vascular corrosion casting3,4. IUGR placenta: Marginal cord insertion was detected. Preliminary results seem to be significantly different from normal placentas as can be seen in Figure 2d, including a reduction of 20% in the number of generations detected; reduction of 87% in the number of bifurcations; and a reduction of 79% in the diameter of the first generation.Summary
- This is the first study to use high resolution ex-vivo MRI to study the human placental vascular architecture.
- Vascular tree characteristics of normal placentas using MRI were in line with the literature.
- A high correlation was found between our method and manual measurements.
- Preliminary results suggest significant differences in vascular tree between normal and IUGR placentas.
Acknowledgements
This work was supported by the Gulton FoundationReferences
1. Rasmussen, A. S., H. Lauridsen, C. Laustsen, B. G. Jensen, S. F. Pedersen, L. Uhrenholt, L. W. Boel, N. Uldbjerg, T. Wang and M. Pedersen (2010). "High-resolution ex vivo magnetic resonance angiography: a feasibility study on biological and medical tissues." BMC Physiol 10: 3.
2. Paul F Whelan (2011) “VSG Image Processing and Analysis (VSG IPA) Toolbox”, Technical Report, Centre for Image Processing & Analysis, Dublin City University.
3. Gordon, Z., D. Elad, R. Almog, Y. Hazan, A. J. Jaffa and O. Eytan (2007). "Anthropometry of fetal vasculature in the chorionic plate." J Anat 211(6): 698-706.
4. Elena Silvia Bernard, Cipria Duta, Sandor Ianos Bernard, Stelian Paneta, Izabella Petre (2016) “Investigation of Chorionic Artery Bifurcation Using Micro Vascular Casting Model” REV.CHIM.(Bucharest) 67 No.2
Figures