4806
Non-invasive placental perfusion imaging in pregnancies complicated by fetal heart disease using velocity-selective arterial spin labeling1Division of Diagnostic Imaging and Radiology, Children’s National Medical Center, Washington, DC, United States, 2Division of Fetal and Transitional Medicine, Children’s National Medical Center, Washington, DC, United States, 3Departments of Radiology and Pediatrics, George Washington University, Washington, DC, United States, 4Department of Radiology, Stanford University, Stanford, CA, United States, 5Division of Cardiology, Children’s National Medical Center, Washington, DC, United States
Synopsis
Recent data have reported that placental dysfunction may be present in the setting of complex fetal congenital heart disease (CHD). We performed placental perfusion imaging using velocity-selective arterial spin labeling in pregnancies complicated by fetal CHD and healthy pregnancies. We demonstrated that global placental perfusion and regional variation of perfusion were significantly correlated with GA in pregnancies complicated by fetal CHD, but not in healthy controls. Our findings suggest that placental ASL may have the potential to serve as an early biomarker of placental dysfunction in fetal CHD.
INTRODUCTION
Brain injury and neurodevelopmental disabilities are prevalent, lifelong complications among surviving infants with congenital heart disease (CHD)1,2. A growing body of evidence suggests that brain injury in CHD may have its origins in fetal life and the causes of CHD are associated with placental dysfunction3-5. However, placental function has been poorly understood namely due to the absence of non-invasive tool for monitoring placental function in utero. In this study we applied 3D velocity-selective arterial spin labeling (VSASL)6 to the placenta to examine placental perfusion in pregnancies complicated by fetal CHD, and healthy controls.METHODS
In the context of a prospective observational study, 12 pregnant women with fetal CHD and 12 healthy pregnancies were recruited for fetal MRI during 2nd or 3rd trimester. All MR imaging was performed on a 1.5 T GE scanner using an 8-channel cardiac coil. In placental ASL, velocity-selective labeling was achieved using dual sech inversion with a cutoff velocity = 2 cm/s, and image acquisition was performed using fast-spin-echo 3D stack of spirals with 8 interleaves, TR/TE = 3000/10 ms, FOV = 36-40 cm, matrix size = 64x64, number of slices = 36-40, and slice thickness = 4 mm. VSASL scan was composed of five pairs of control/labeled imaging followed by proton-density imaging, leading to total scan time of 4:29 min. Global placental perfusion was estimated by averaging perfusion in the whole placenta that was manually segmented on each image. To estimate regional perfusion variation, the coefficient of variation (i.e. standard deviation/mean) of segmented perfusion (1.5 x1.5 voxels) in the whole placenta was calculated.RESULTS
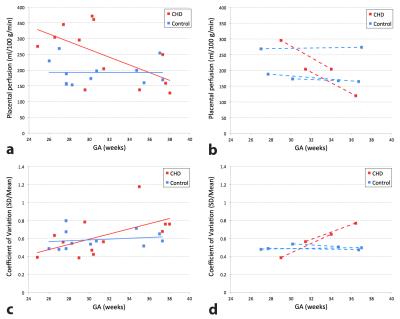
All enrolled participants were included in our analysis. Mean gestational age (GA) was 31 ± 4 weeks for both CHD and controls (p=0.60). Diagnostic categories for fetal CHD included: hypoplastic left heart syndrome (n=5), tetralogy of Fallot (n=3), ventricular septal defect (n=2), and double-outlet right ventricle (n=1). Global placental perfusion was found to be significantly higher (248 ± 92 ml/100 g/min) in fetal CHD compared to healthy controls (193 ± 40 ml/100 g/min) (p=0.03); however we did not detect a similar difference in regional perfusion between fetal CHD and controls (p=0.61). Both global perfusion and regional perfusion variation were significantly correlated with GA in pregnancies with CHD fetuses (r=-0.60, p=0.04 and r=0.58, p=0.04 respectively), but not in healthy controls (r=-0.01, p=0.98 and r=0.19, p=0.55 respectively) (see Figures 1a,c). In addition, 2 out of 12 pregnant women with fetal CHD and 3 out of 12 healthy pregnant women underwent a second, follow-up fetal MRI. Figures 1b,d depict only the data from these five subjects. The slopes between the two data points within each subject were similar to those of linear regression lines of their own group (either CHD or controls).CONCLUSION
We report for the first time, altered placental perfusion in fetal CHD compared with healthy controls using VSASL. Our preliminary results show that global placental perfusion decreases and regional variation of perfusion increases with advancing GA in pregnancies complicated by fetal CHD while healthy pregnancies show no correlation between placental perfusion and GA. These data suggest that placental ASL may serve as a potential early biomarker of placental dysfunction in fetal CHD that may worsen throughout gestation.Acknowledgements
No acknowledgement found.References
1. Donofrio MT, Duplessis AJ, Limperopoulos C. Impact of congenital heart disease on fetal brain development and injury. Curr Opin Pediatr. 2011;23:502-511.
2. Massaro AN, El-Dib M, Glass P, et al. Factors associated with adverse neurodevelopmental outcomes in infants with congenital heart disease. Brain Dev. 2008;30:437– 446.
3. Huhta J, Linask KK. Environmental origins of congenital heart disease: the heart-placenta connection. Semin Fetal Neonatal Med 2013;18:245-250.
4. Andescavage N, Yarish A, Donofrio M, et al. 3-D volumetric MRI evaluation of the placenta in fetuses with complex congenital heart disease. Placenta 2015;36:1024-1030.
5. Jones HN, Olbrych SK, Smith KL, et al. Hypoplastic left heart syndrome is associated with structural and vascular placental abnormalities and leptin dysregulation. Placenta 2015;36:1078-1086.
6. Wong EC, Cronin M, Wu WC, et al. Velocity-Selective Arterial Spin Labeling. Magn Reson Med 2006;55:1334–1341.
Figures