4801
Perfusion MRI of the Placenta: Preliminary Results using ASL FAIR and Ferumoxytol DCE MRI in the Rhesus Macaque1Medical Physics, University of Wisconsin - Madison, Madison, WI, United States, 2Radiology, University of Wisconsin - Madison, Madison, WI, United States, 3Biomedical Engineering, University of Wisconsin - Madison, Madison, WI, United States, 4Comparative Biosciences, University of Wisconsin - Madison, Madison, WI, United States, 5Obstetrics and Gynecology, University of Wisconsin - Madison, Madison, WI, United States, 6Medicine, University of Wisconsin - Madison, Madison, WI, United States, 7Emergency Medicine, University of Wisconsin - Madison, Madison, WI, United States
Synopsis
Non-contrast enhanced methods are needed to quantify placental perfusion to detect and monitor pathophysiological changes during pregnancy. We evaluate the potential of an endogenous perfusion labeling perfusion technique, Arterial Spin Labeled Flow-sensitive Alternating Inversion Recovery (ASL FAIR), and compare with ferumoxytol dynamic contrast enhanced (DCE) MRI. Three pregnant rhesus macaque were imaged with both FAIR and ferumoxytol DCE. Localized regions of ASL perfusion were observed that coincided with regions of early contrast enhancement seen in DCE. Ferumoxytol DCE measured perfusion with extended transit times across the placenta beyond those typically used for ASL labeling.
Introduction
The placenta supplies the fetus with nutrients and oxygen via exchange between the maternal and fetal blood. Perfusion deficiencies impede nutrient exchange, affecting placental function and fetal health.1 Tools are needed for in vivo quantitative assessment of placental vascular function in utero.2 Non-contrast Arterial Spin Labeled (ASL) MRI methods are a promising approach for safe evaluation of placental perfusion.3 However, the maternal and fetal vascular network within the placenta is unique which may confound the interpretation of the ASL signal. Here, we evaluate the potential of two variants of ASL Flow-sensitive Alternating Inversion Recovery (FAIR) to assess intervillous perfusion and compare with ferumoxytol-enhanced Dynamic Contrast Enhanced (DCE) MRI in the rhesus macaque.Methods
All procedures were approved by the institution’s animal care and use committee (IACUC). Three pregnant rhesus macaques (2 healthy controls and 1 with IL-1β amniotic fluid infusion to produce an inflammatory response) were imaged under isoflurane sedation in the right lateral position at gestational ages of 106 (Rhesus #1), 91 (Rhesus #2), and 93 days (Rhesus #3 - infection), all late second trimester.
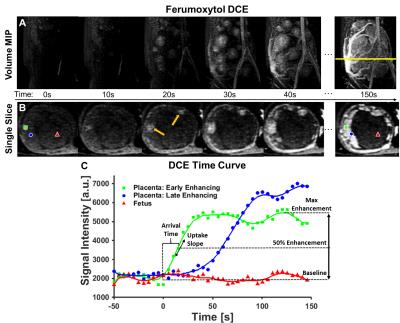
MRI was performed at 3.0T (Discovery MR750, GE Healthcare, Waukesha, WI) with a 32-channel phased array coil (Neocoil, Pewaukee, WI). A 2D respiratory-triggered ASL FAIR acquisition with SSFSE readout of a single 4mm slice using a 2.0s post-label delay (TI), TR/TE=~6.6s/49.2ms, FOV=18cm2, and 20 control/tag image pairs. Additionally, images were collected with outer volume suppression (OVS) pulses added to the ASL FAIR acquisition to reduce the macrovasculature signal. OVS pulses (80mm thick, 90° flip) were applied 1.0s after the FAIR preparation above and below the ASL imaging slice. DCE data were acquired in the same animals during intravenous injection of ferumoyxtol (FerahemeTM, AMAG Pharmaceuticals, Waltham, WI). 4mg ferumoyxtol/kg bodyweight was diluted 5:1 with saline and infused over 20 seconds. A respiratory gated 3D T1-weighted spoiled gradient echo (DISCO) sequence acquired dynamic volumetric data (TR=4.8ms, FOV=22cm x 15.4cm, 68 slices 2mm thick, 5.0s temporal resolution, flip angle=12°). All image post-processing was performed in Matlab R2014b (Mathworks, Natick, MA). The DCE data was resampled to spatially match the ASL FAIR data from which arrival time, uptake slope, and max enhancement maps were generated on a voxel-wise basis. Parameter derivation is explained graphically in Figure 2 using an example uptake curve. Arrival time was defined as the time to 50% maximum enhancement and the uptake slope was measured at this 50% enhancement point.
Results
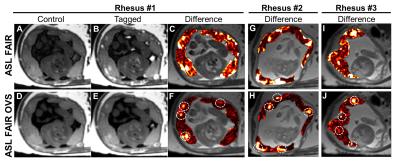
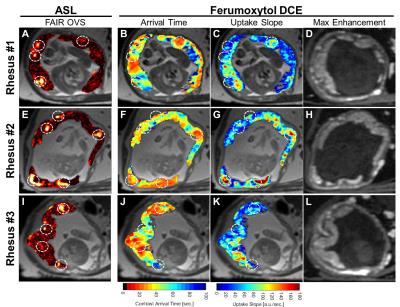
The application of OVS to ASL FAIR results in an overall reduction in vascular signal as intended with remaining regions of high local perfusion indicated by white dotted circles, for the three animals studied (Figure 1). Maximum intensity projections and single slice images at corresponding time points demonstrate dynamic ferumoxytol contrast arrival (Figure 2). The large range of arrival times to the intervillous space are clearly depicted in the representative DCE kinetic curves. This is further supported by the model-free parametric maps derived from DCE data (Figure 3). The arrival time map, in particular, depicts extended transit times across the placental region [1-60s] with early contrast arrival regions coinciding with high ASL perfusion signal and high contrast uptake slope. No clear qualitative or quantitative differences in perfusion were observed between the healthy controls and the induced inflammation model (Rhesus #3) in this ongoing study.Discussion
In this study, we imaged the placenta of three pregnant rhesus macaques using ASL FAIR and DCE MRI perfusion techniques and performed a comparative analysis. While placental perfusion has been investigated in human studies with ASL,3,4 comparisons in animal studies with reference standards are limited. The regions of intense perfusion are likely the maternal utero-placental spiral arteries entering the intervillious space since focal placental blood delivery is visible on both ASL FAIR and in the early-arrival time frames in ferumoxytol DCE. The heterogeneous nature of intervillious perfusion signal in FAIR suggests that voxel-wise interpretation at distal regions from the spiral arteries may be confounded by the limits of the FAIR inversion tagging duration and delay. This observation is corroborated by the extended transit time across the placental region from ferumoxytol DCE images similar to that observed by others with Gd-based DCE MRI.5Conclusion
ASL FAIR and ferumoxytol DCE are feasible to detect early blood delivery to the placenta in a pregnant rhesus macaque model. An endogenous labeling perfusion technique is potentially advantageous for safety and cost reasons when translating to human mothers but is possibly limited due to the extended transit times for placental tissues beyond the immediate vicinity of the maternal utero-placental spiral arteries.Acknowledgements
The authors thank our collaborators and colleagues. We gratefully acknowledge the NIH Human Placental Project (NICHD U01HD087216), NIH grant number P51 OD011106 to the Wisconsin National Primate Research Center, NIH awards K24 DK102595, UL1TR000427 and TL1TR000429, UW School of Medicine and Public Health and UW Departments of Medical Physics, Radiology, and Obstetrics and Gynecology and GE Healthcare for research support.References
1Krishna U, Bhalerao S. Placental Insufficiency and Fetal Growth Restriction. Journal of Obstetrics and Gynaecology of India. 2011;61(5):505-11.
2Guttmacher AE, Maddox YT, Spong CY. The Human Placenta Project: placental structure, development, and function in real time. Placenta. 2014;35(5):303-4.
3Derwig I, Lythgoe DJ, Barker GJ, Poon L, Gowland P, Yeung R, et al. Association of placental perfusion, as assessed by magnetic resonance imaging and uterine artery Doppler ultrasound, and its relationship to pregnancy outcome. Placenta. 2013;34(10):885-91.
4Gowland PA, Francis ST, Duncan KR,
Freeman AJ, Issa B, Moore RJ, et al. In vivo perfusion measurements in the
human placenta using echo planar imaging at 0.5 T. Magnetic Resonance in
Medicine. 1998;40(3):467-73.
5Frias AE, Schabel MC, Roberts VHJ, Tudorica A, Grigsby PL, Oh KY, et al. Using Dynamic Contrast-Enhanced MRI to Quantitatively Characterize Maternal Vascular Organization in the Primate Placenta. Magnetic Resonance in Medicine. 2015;73(4):1570-8.
Figures