4800
DECIDE: Diffusion-rElaxation Combined Imaging for Detailed Placental Evaluation1University College London, London, United Kingdom, 2Institute for Women's Health, University College Hospital, London, United Kingdom, 3Medical Physics, University College Hospital, London, United Kingdom, 4Centre for Medical Imaging, University College Hospital, London, United Kingdom, 5UZ Leuven
Synopsis
We propose a new multi-compartment model for the tissue signal in MRI and apply this to images of liver and placenta. Motivated by different flow characteristics in these organs, a three compartment model comprising fast and slowly circulating fluid pools and a tissue pool is fitted to overlapping multi-echo T2 relaxometry and an intra-voxel incoherent motion diffusion acquisition with low b-values. We compare and contrast parametric maps for regions of interest in liver and placenta.
Introduction
The placenta performs a vital role in the transfer of oxygen, nutrients and waste products between the fetus and the mother by maintaining two distinct circulatory systems. The flow properties of this tissue are unique with a rapidly perfusing fetal capillary network bathed within a large slow moving maternal circulation. The placenta is vulnerable to a number of pathologies and there is growing interest in monitoring placental function using MRI [1]. Several contrasts have been investigated for monitoring placental blood flow including intra-voxel incoherent motion model (IVIM, [2]) and T2 relaxometry, [3,4]. However, the effects seen using both IVIM and T2 relaxometry are dependent upon one another and it can be difficult to isolate changes to the structural T2 measurement from the flow measurement of IVIM. In this work we outline a novel three-compartment model that combines IVIM and T2 relaxometry that we hypothesise represents the different vascular compartments in the placenta. We compare the results of model-fitting in six free-breathing livers and three free-breathing placentae.Materials
On a 1.5T Siemens Symphony, we obtained six free-breathing liver datasets and three free-breathing placenta datasets at an approximate gestation 24 weeks. We obtained IVIM measurements at seven b-values [0,50,100,150,200,400,600]$$$s.mm^{-2}$$$and T2 relaxometry measurements at 9 echo times [77,90,120,150,180,210,240,270,300]$$$ms$$$ at resolution $$$1.9x1.9x6mm$$$. The b-value increments and shortest TE were limited by the MR scanner. In addition we obtained T2 relaxometry measurements at the same 9 echo times with a diffusion b-value of $$$b=200s.mm^{-2}$$$ to better separate long T2 compartments with different incoherent motion properties. The total acquisition time was about 20 minutes. We used a non-rigid registration routine to align all images [5].Methods
We propose a general three-compartment model that distinguishes rapidly moving (perfusing) fluid with high pseudo-diffusivity; slowly-moving or static fluid with high T2; and otherwise static tissue with low-diffusivity and low T2. These are given respective volume fractions $$$f$$$ for moving fluid and $$$\nu$$$ for static fluid. The signal model for $$$S$$$, as a function of echo-time $$$t$$$ and diffusion weighting $$$b$$$, is represented by equation 1 combining the effects of T2 relaxation and diffusion-weighting. $$$S_0$$$ represents the initial signal magnitude. The diffusivities of these compartments are given by $$$d^*$$$ for moving fluid and $$$d$$$ for the remaining fluid and contribution of tissue since typically $$$d^*$$$ is much larger than $$$d$$$. We fix the relaxivities from literature values: $$$r_2^b=295ms$$$ for fluid and $$$r_2^t=60ms$$$ for tissue [6]. This is equivalent to the standard IVIM model if $$$r_2^b=r_2^t$$$ and $$$\nu=0$$$, although this changes the interpretation of the perfusion fraction $$$f$$$.
$$S(b,t)=S_{0}\bigl[ f e^{-\textbf{b}d^*-\textbf{t}r_2^b} + (1-f) e^{-\textbf{b}d} \bigl( \nu e^{-\textbf{t}r_2^b} + (1-\nu) e^{-\textbf{t}r_2^t} \bigr) \bigr]$$
We compare our proposed multi-compartment model with existing models. We evaluate the apparent diffusion coefficient (ADC), and the single-compartment T2 rate. Standard IVIM [2] simultaneously estimates the volume fraction of two compartments of diffusion, associating high-diffusivity regions with perfusing or moving fluid; thus we also evaluate the standard IVIM perfusion fraction. Results are presented as histograms over all voxels within manually segmented regions of interest.
Results
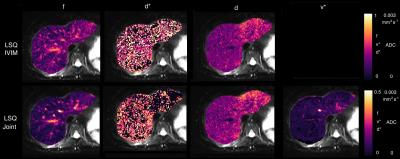
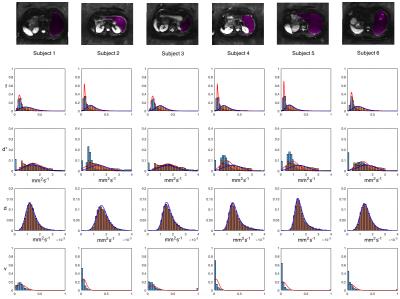
Parametric maps for the liver are shown in Figure 1 for parameters drawn from standard IVIM (top row) and the three-compartment model (bottom row). Any contribution from static fluid, $$$\nu$$$ is minor. Figure 2 shows ROI histograms for six liver datasets for volume fractions and diffusivities. IVIM results are shown with orange bars, 3-compartment results with blue bars. There is little support across all datasets for a significant non-moving fluid compartment, consistent with known properties of the liver.
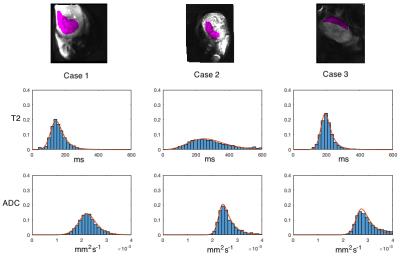
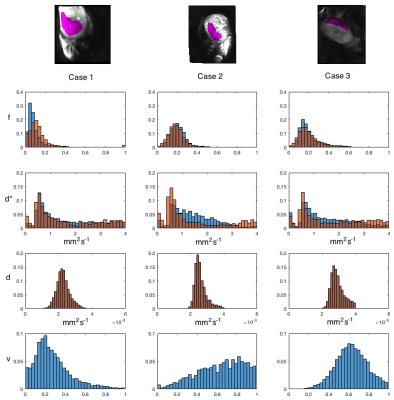
Placenta parameter histograms are shown in Figure 3 for single-compartment ADC and T2. Figure 4 compares histogram distributions of IVIM (orange) with the three-compartment model (blue). A significant non-zero volume fraction $$$\nu$$$ is seen for static/slowly moving fluid. Perfusion fractions remain comparable between the two models.
Discussion
The multi-modal data presented in this work have allowed a new model to be applied to combined perfusion, diffusion and relaxometry images, specifically for use in placenta image analysis where different blood flow regimes are present. The model was used in two applications: within the liver to show comparable results to existing methods; and within the placenta. In contrast to the liver, we show that there is evidence of a large slowly-moving or static fluid pool within the placenta and the separation of this component from the IVIM signal is important for disentangling the signal coming from a conventional IVIM acquisition. The identification of both slowly moving and rapidly moving fluid (blood) pools could be of interest for assessment of unique placental pathologies.Acknowledgements
We would like to acknowledge the MRC (MR/J01107X/1), the National Institute for Health Research (NIHR), the Wellcome Trust, the EPSRC (EP/H046410/1,NS/A000027/1) and the National Institute for Health Research University College London Hospitals Biomedical Research Centre (NIHR BRC UCLH/UCL High Impact Initiative BW.mn.BRC10269). This work is supported by the EPSRC-funded UCL Centre for Doctoral Training in Medical Imaging (EP/L016478/1).References
[1] Penny Gowland. Placental MRI. Semin Fetal Neonatal Med, 10(5):485–490, Oct 2005.
[2] D. Le Bihan, E. Breton, D. Lallemand, M. L. Aubin, J. Vignaud, and M. Laval-Jeantet. Separation of diffusion and perfusion in intravoxel inco- herent motion mr imaging. Radiology, 168(2):497–505, Aug 1988.
[3] I. Derwig, G. J. Barker, L. Poon, F. Zelaya, P. Gowland, D. J. Lythgoe, and K. Nicolaides. Association of placental t2 relaxation times and uterine artery doppler ultrasound measures of placental blood flow. Placenta, 34(6):474–479, Jun 2013.
[4] I. Derwig, D. J. Lythgoe, G. J. Barker, L. Poon, P. Gowland, R. Yeung, F. Zelaya, and K. Nicolaides. Association of placental perfusion, as assessed by magnetic resonance imaging and uterine artery doppler ultrasound, and its relationship to pregnancy outcome. Placenta, 34(10):885–891, Oct 2013.
[5] A. Melbourne, D. Atkinson, M. J. White, D. Collins, M. Leach, and D. Hawkes. Registration of dynamic contrast-enhanced MRI using a progressive principal component registration (PPCR). Phys Med Biol, 52(17):5147– 5156, Sep 2007.
[6] Greg J. Stanisz, Ewa E. Odrobina, Joseph Pun, Michael Escaravage, Simon J. Graham, Michael J. Bronskill, and R Mark Henkelman. T1, T2 relaxation and magnetization transfer in tissue at 3T. Magn Reson Med, 54(3):507–512, Sep 2005.
Figures