4796
Quantitatively Differentiating Prostate Cancer, Prostatitis and Benign Prostatic Hyperplasia (BPH) by Diffusion MRI Histology (D-Histo)1Chemistry, Washington University, St. Louis, MO, United States, 2Radiology, Changhai Hospital, Shanghai, People's Republic of China, 3Radiology, Washington University, St. Louis, MO, United States, 4Urology, Changhai Hospital, Shanghai, People's Republic of China, 5Biomedical Engineering, Washington University, St. Louis, MO, United States, 6Biology, Washington University, St. Louis, MO, United States, 7Hope Center for Neurological Disorder, Washington University, St. Louis, MO, United States
Synopsis
A recent consensus established mpMRI for PCa detection has not had the needed sensitivity or specificity to distinguish prostatitis from PCa. Thus, PCa diagnosis by MRI remains uncertain, resulting in over-diagnosis and over-treatment. For the first time, we demonstrate that the new method, diffusion MRI histology (D-Histo), accurately localizes and quantifies PCa, prostatitis, and BPH. With improved diagnosis accuracy, D-Histo affords effective guidance of treatment planning, and assessment of treatment efficacy.
Introduction
Prostate cancer (PCa) is the most prevalent malignancy afflicting American.1 While ultrasound-guided needle biopsy is the clinical standard for prostate cancer diagnosis, it misses 20-30% of clinically significant tumors. Multiparametric MRI (mpMRI) has been widely used to detect clinically significant PCa and guide biopsies. However, prospective evaluation of PCa diagnoses using mpMRI suggests a significant false positive rate reducing the effectiveness of cancer detection using mpMRI.2 Current imaging modalities, including mpMRI, do not distinguish PCa from prostatitis or stromal BPH. The contribution of inflammatory cells or BPH to the prostate ADC measurement has not been definitively established. Herein, we demonstrate inflammatory (~ 5 µm) and cancer cells (≥ 20 µm) in prostate can be detected, distinguished, and quantified via their signature restricted-isotropic diffusion with our newly-developed Diffusion MRI Histology (D-Histo) technique. In addition, the D-Histo-derived anisotropic-diffusion-tensor fraction provides a means to detect and quantify stromal BPH.Methods
Patient recruitment: Between May 2015 and July 2016, 181 patients with clinical suspicion of prostate cancer and without any preoperative treatment were recruited and scheduled for mpMRI and D-Histo scan in Changhai Hospital, Shanghai, China. This study was approved by the local Institutional Review Board.
MRI and image analysis: In vivo diffusion-weighted MRI data was obtained on a 3T Siemens Skyra scanner (Erlangen, Germany) with an 18-channel phased-array body receive coil. Single-shot echo-planar diffusion weighted imaging was performed in transverse view (2×2×4 mm3) using a 25-direction encoding scheme (maximum b-value of 1500 s/mm2) in 8 minutes. Both D-Histo and DTI analyses were performed using an in-house software. Standard mpMRI including T1-weighted, T2-weighted, diffusion-weighted imaging, dynamic contrast-enhanced pulse sequences were obtained in all patients. Prostate Imaging Radiology Assessment and Diagnostic System (PIRADS) was used by experienced radiologists for assessing prostate cancer suspicion on mpMRI.
Histology: After prostatectomy, whole mount section was performed. Sectioned slabs were then completely embedded in paraffin and sectioned in 4-µm thick for hematoxylin and eosin staining (H & E).
Results and Discussion
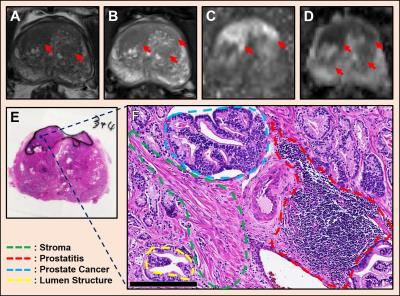
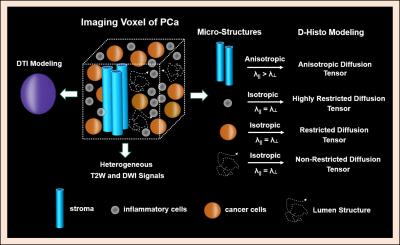
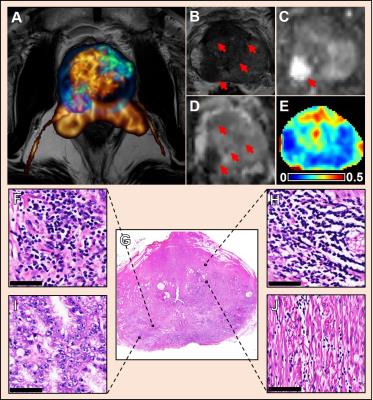
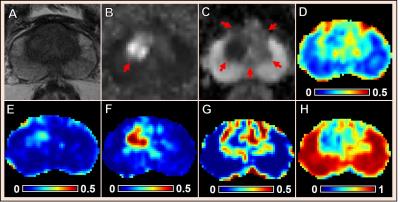
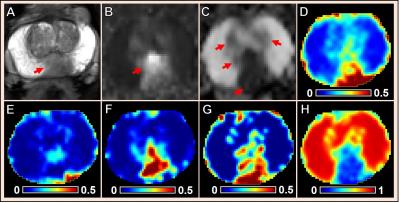
Multiple coexisting pathologies and structures (e.g., stromal BPH, prostatitis, and/or lumen water in addition to PCa) are common within prostate gland, leading to false-positive PCa diagnosis using mpMRI alone (Fig. 1). Although the postmortem histology remains the “gold standard” PCa diagnosis, the expanded pathologist-identified cancer region actually contains coexisting PCa, inflammation (prostatitis) and stroma (Fig. 1). We modified previously developed diffusion basis spectrum imaging (DBSI)3-5 to more accurately depict coexisting prostate pathologies and structures (Fig. 2), removing multiple-fiber consideration and improving isotropic diffusion tensor definition. D-Histo differentiates and quantifies stroma/BPH as an anisotropic-diffusion tensor, inflammatory cells as highly-restricted-isotropic-diffusion tensor, PCa as a restricted-isotropic-diffusion tensor with lesser restriction, and normal prostate a non-restricted-isotropic-diffusion tensor (representative of lumen water). A 3D-rendered prostate from D-Histo overlaid on an anatomical T2WI was obtained from a 58-year old patient with PSA level of 16.49 ng/mL with PCa grading by needle biopsy (Gleason scorer 3 + 4) and prostatectomy (pathological stage of T3A). Suspected PCa located at the peripheral zone (right lateral anterior; pink) with transition zone BPH (gold) and peripheral zone inflammation (blue-green). The H&E staining of corresponding whole-mount-section slide (Fig. 3F) supports the results seen by D-Histo, including discovery of chronic prostatitis (Fig. 3G, HH), PCa (Fig. 3I), and stromal BPH (Fig. 3J). Complications of mpMRI in detecting transition zone PCa is demonstrated (Fig. 4) where T2WI was ambiguous (Fig. 4A), DWI underestimated PCa (Fig. 4B), and ADC map overestimated PCa (Fig. 4C). D-Histo distinguished and quantified overlapping prostatitis (Fig. 4E) and PCa (Fig. 4F) that were surrounded by stromal BPH (Fig. 4G). The high hindered isotropic diffusion fraction revealed normal peripheral zone prostate tissues (Fig. 4H). Another representative case with ambiguous transition zone T2W (Fig. 5A) and ADC (C) complicated by the mixture of prostatitis (Fig. 5E), PCa (Fig. 5F) and BPH (Fig. 5G). DTI-FA failed to correctly reflect stroma (Fig. 5D) as the anisotropic diffusion was averaged by the isotropic diffusion of the coexisting PCa and inflammation cells.Conclusion
For the first time, co-existing PCa, prostatitis and stromal BPH have been quantitatively differentiated noninvasively using D-Histo, verified by whole mount histology. Accurate localization and quantification of prostate pathologies and structures by D-Histo resolves the unmet needs in PCa imaging.Acknowledgements
No acknowledgement found.References
1. Society AC. Cancer Facts & Figures 2016. 2016; http://www.cancer.org/research/cancerfactsstatistics/cancerfactsfigures2016/index.
2. Mertan FV, Greer MD, Shih JH, et al. Prospective Evaluation of the Prostate Imaging Reporting and Data System version 2 (PI-RADSv2) for Prostate Cancer Detection. J Urol. Apr 18 2016.
3. Chiang CW, Wang Y, Sun P, et al. Quantifying white matter tract diffusion parameters in the presence of increased extra-fiber cellularity and vasogenic edema. NeuroImage. Nov 1 2014;101:310-319.
4. Wang Y, Sun P, Wang Q, et al. Differentiation and quantification of inflammation, demyelination and axon injury or loss in multiple sclerosis. Brain : a journal of neurology. May 2015;138(Pt 5):1223-1238. 5. Wang Y, Wang Q, Haldar JP, et al. Quantification of increased cellularity during inflammatory demyelination. Brain : a journal of neurology. Dec 2011;134(Pt 12):3590-3601.
Figures