4778
Role of DWI in guiding MRI-TRUS fusion biopsy in patients with prostate cancer: a prospective cohort study1Radiology, All India Institute of Medical Sciences, New Delhi, India, NewDelhi, India, 2Uology, All India Institute of Medical Sciences, New Delhi, India, NewDelhi, India, 3Urology, All India Institute of Medical Sciences, New Delhi, India, NewDelhi, India, 4Pathology, All India Institute of Medical Sciences, New Delhi, India, NewDelhi, India
Synopsis
Transrectal ultrasound (TRUS) guided 12 core biopsy of prostate has a sensitivity of 39-52%. We prospectively evaluated the role of DWI in guiding MRI-TRUS fusion biopsy. MRI was performed on a 3 Tesla system. PIRAD score was assigned and PIRAD 3-5 score were subjected to targeted fusion biopsy using the Artemis device along with standard 12 core biopsies. Targeted biopsy detected a higher number (93%) of clinically significant cancers and 71% cancers were upgraded to significant cancer on targeted biopsy. Fusion biopsies guided by DWI thus provide incremental information over standard TRUS biopsies in the diagnosis of significant prostate cancer.
Introduction
Transrectal ultrasound (TRUS) guided 12 core biopsy of prostate, the current standard, has a sensitivity of 39-52% in diagnosing prostate cancer. MRI TRUS fusion biopsies based on diffusion weighted imaging (DWI) and apparent diffusion coefficient (ADC) map increase accuracy of biopsies and may improve their yield. We prospectively evaluated the role of DWI in guiding MRI-TRUS fusion biopsy in a cohort of men with suspicion of prostate cancer.Materials and Methods
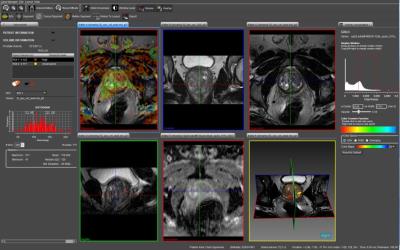
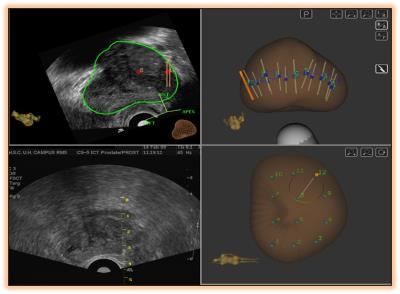
In an IRB approved prospective cohort study, 100 men with suspicion of prostate cancer were recruited to undergo an MRI-TRUS fusion biopsy using the Artemis(R) (Eigen, USA) device. MRI was performed on a 3 Tesla system (Achieva, Philips Healthcare, Best, Netherlands) using a 16 channel phased array torso coil. T2W Turbo spin echo (TSE) fat suppressed images, T1W TSE non-fat suppressed images and free breathing Echo planar imaging (EPI) based DWI sequence using b values of 0, 500, 1000 and 1500. Subsequently, the T2 axial and ADC images were analysed using the PROFUSE software. A 3-D prostate model was generated and freehand regions of interest (ROI) were manually drawn around the suspicious areas for PIRAD score assignment. The processed data were then uploaded on the Artemis(R) (Eigen, USA) device before beginning the biopsy. A sterile urine culture was ensured for all patients scheduled for biopsy, and suppressive antibiotics were administered to those on indwelling Foley’s catheters or to those with repeated mixed growth on urine culture. Anticoagulants were stopped for at least 5-7 days prior to and after biopsy, in accordance with the cardiological consultation. Biopsy was performed in the left lateral position with the hips lying at edge of the couch. Following introduction of the USG probe (BK 8818 probe, 12 MHz of BK Medicals, USG device), infiltrative local anaesthesia (Xylocaine without adrenaline 2%, diluted to 1:1, 10 ml each on either side) were administered each side at base of prostate, just at prostate-seminal vesicles angle. MRI images were fused to the real time TRUS images on the Artemis device. A final plan was created displaying the systematic (Random) 12 cores as well as the targeted core(s) lying within the ROIs. For biopsy the standard Bard’s biopsy gun (18 G/25 cm) was used for each patient Single core was taken from each of the Systematic 12 cores. At least 2 cores (depending upon the area of the ROI) were taken from each of the targeted region (s) assigned PIRAD score of 3-5. Samples were immediately sent for histopathological evaluation in a specifically marked and numbered sterile formalin vials. Yield from standard cores was compared with targeted cores. Gleason scores of 4+3 or higher were considered as significant prostate cancer. The Data was analysed using statistical software, STATA version 14.0. Quantitative data was expressed as mean, Standard deviation, and median (minimum-maximum) as appropriate. Qualitative data was expressed as frequency and percentage. Fisher’s exact test was used to check the association between the variables. Student t test and Mann Whitney U test was used to compare normal and skewed variables respectively. p value ≤ 0.05 was considered as statistically significant.Results
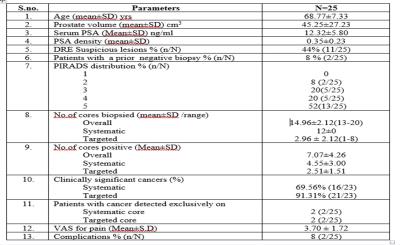
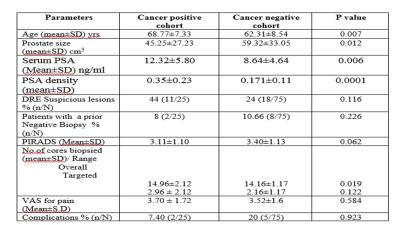
Among 100 patients, mean age of the study population was 64.06 years (Range 41-82). The mean prostate volume was 55.52 cc (range 15-191). The mean S.PSA and PSA-Density was 9.63 ng/ml (Range 0.41-11.93) and 0.22 (0.22 ±0.17) respectively. The mean PIRADS score was 3.33. The most frequent lesion among the study group was PIRADS 3 which occurred in 30 (30/100) patients. 10 (10/100) patients had a history of a previous negative standard 12 core TRUS guided biopsy on one or more occasion. The mean number of core biopsied was 14.38 (Range 13-20) and mean number of targeted cores biopsied were 2.38 (Range 1-8). 25 patients had cancer of which 2 (8%) were detected only on standard cores and 2 (8%) only on targeted cores. Of the clinically significant prostate cancers, targeted biopsy detected a higher number (21/23, 93%) than standard biopsy (16/23, 69%). 5 of 7 (71%) cancers that were insignificant on standard biopsy were upgraded to significant cancer on targeted cores. The mean VAS for pain in the study population was 3.5. The overall complication rate was 7% (7/100) of which 5 had post biopsy fever requiring admission and parenteral antibiotics.Conclusion
8% cancers were detected only on MRI-TRUS fusion targeted biopsies guided by DWI while it upgraded more than two-thirds of insignificant cancers to significant cancers. Fusion biopsies guided by DWI thus provide incremental information over standard TRUS biopsies in the diagnosis of significant prostate cancer.Acknowledgements
No acknowledgement found.References
[1] Hoeks CM, Schouten MG, Bomers JG et al: Three-Tesla magnetic resonance-guided prostate biopsy in men with increased prostate-specific antigen and repeated, negative, random, systematic,transrectal ultrasound biopsies: detection of clinically significant prostate cancers. Eur Urol, Epub ahead of print February 1,2012
[2] Vourganti S, Rastinehad A, Yerram NK, et al. Multiparametric magnetic resonance imaging and ultrasound fusion biopsy detecprostate cancer in patients with prior negative transrectal ultrasound biopsies. J Urol 2012;188:2152–7.
[3] Sonn GA, Chang E, Natarajan S, et al. Value of targeted prostate biopsy using magnetic resonance-ultrasound fusion in men with prior negative biopsy and elevated prostate-specific antigen. Eur Urol. In press.
[4] Magnetic Resonance Imaging/Ultrasound–Fusion Biopsy Significantly Upgrades Prostate Cancer Versus Systematic 12-core Transrectal Ultrasound Biopsy M. Minhaj Siddiqui, Soroush Rais-Bahramia, Hong Truong a, Lambros Stamatakis , Srinivas Vourganti , Jeffrey Nix , Urologic Oncology Branch, National Cancer Institute
[5] Vourganti S, Rastinehad A, Yerram NK, et al. Multiparametric magnetic resonance imaging and ultrasound fusion biopsy detect prostate cancer in patients with prior negative transrectal ultrasound biopsies. J Urol 2012;188:2152–7.