4777
Target performance of MRI-ultrasound fusion guided prostate biopsy in a cohort of patients suspicious for prostate cancer1Pharmaceutical sciences institute, ETHZ, Zurich, Switzerland, 2Zentrum für Urologie, Hirslanden Klinik, Zurich, Zurich, Switzerland, 3Institut für histologische und zytologische Diagnostik, Aarau, Switzerland, 4Radiologie, Hirslanden Klinik, Zurich, Zurich, Switzerland
Synopsis
Conventional systematic core biopsies might fail to detect clinical significant prostate cancer. ARTEMIS MRI-ultrasound fusion guided prostate biopsy (ART-PBx) might overcome this issue by improving the targeting of suspicious lesions. In a retrospective clinical study including 194 patients (243 lesions) we determined a target performance of 56.3 % positive biopsies related to histopathology. The detection rate rises up to 71.4% for high score lesions (PIRADS 5) but did not show any correlation with lesion’s size. This target methodology based on MRI achieves greater detection rate of clinical significant prostate cancer, improving the ability to appropriately counsel patients regarding therapy.
Purpose
Prostate needle biopsy, when performed by the conventional method, may fail to detect the presence of cancer1. Technical improvements in prostate magnetic resonance imaging (MRI) have conducted to the use of MRI to target prostate biopsies2. Fusion of MRI images with ultrasound allows urologists to progress from conventional core systematic biopsies to targeted biopsies3. The technology involves fusion biopsy, wherein the MRI features are combined with ultrasound guidance in a traditional urologic biopsy suite. Recent evidence support the high potential of targeted biopsies which have shown to be more accurate as systematic biopsies alone4. Such a fusion device (Artemis, Eigen, Grass Valley, CA, USA) has been approved by the FDA and might improve the efficiency of prostate biopsy. In the present study, we sought to assess the target performance of the ARTEMIS MRI-ultrasound fusion guided prostate biopsy (ART-PBx) procedure to detect clinical significant prostate cancer based on histopathological results.Methods
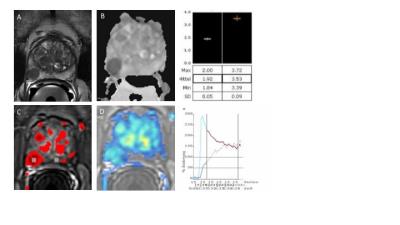
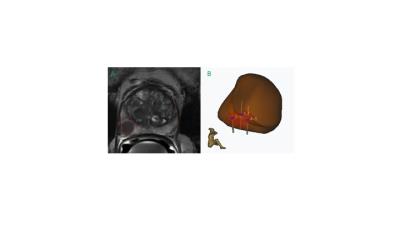
In a single-center study we retrospectively reviewed 245 patients at early stage of prostate cancer who underwent in total 293 biopsies between August 2015 and July 2016. The subjects included in the study presented an elevated serum PSA level or serum PSA level rise and or an abnormal rectal examination. Subsequently, a standardized multi-parametric 3T MRI examination including 3D T2-weighted sequence, diffusion weighted imaging, ADC map and dynamic perfusion analysis (in- and outflow) was performed in search for suspicious cancer lesions (see figure 1). Lesions were characterized by size, localisation according to the Dickinson’s scheme and PIRADS score. Patients with significant PIRADS grades (3, 4 or 5) underwent a standardized ART-PBx procedure using the previously acquired MR images. Briefly, T2 MRI images with delineated prostate and supicious lesions were introduced into the ARTEMIS device. A conventional transrectal ultrasound device acquired real time images of the prostate. Fusion with MRI data was carried out and the preselected biopsy sites were collected (see figure 2). Finally, histopathological findings for clinical significant prostate cancer (Gleason score, proliferation rate and percentage of affected tissue in the cutting cylinder) were correlated with initial MRI findings indicating the target performance of the ART-PBx procedure. Furthermore, the target performance was subanalyzed for the single lesions based on their initial PIRADS classification (excluding unclassified lesions) and correlated with their size. Descriptive statistics were used to summarize patient characteristics such as age and serum PSA level. Correlations between continuous variables were made using the nonparametric Spearman rank correlation.Results
The final study population consisted of 194 patients fulfilling all inclusion and exclusion criteria. At fusion biopsy, the median PSA level was 6.61 ng/ml (IQR: 4.7 to 9.36) and the median age was found to be at 69 years (IQR: 62 to 72). 129 lesions out of 243 suspicious lesions were positive with clinical significant prostate cancer based on histopathology yielding an overall ART-PBx target performance rate of 53.1%. On a patient level, 56.3% of 194 patients were positive. Subanalysis of the PIRADS criteria delivered an escalating target performance increasing significantly in parallel with PIRADS scoring (PIRADS 3: 14,7%, PIRADS 4: 39.8%, PIRADS 5: 71.4% of all lesions, respectively). No significant correlation was found between lesion size and histopathological results (r = 0.09, p = 0.19).Discussion and Conclusion
In the present study, in men with suspicion for prostate cancer Artemis MRI-ultrasound fusion guided prostate biopsy yielded a 56.3% cancer-detection rate based on histopathology. With high PIRADS score (PIRADS5) the detection rate of clinical significant prostate cancer increased up to 71.4% whereas a poor performance is achieved with low PIRADS grade (PIRADS3). These results are consistent with previously published data emphasizing the clinical importance of MRI to guide biopsy, notably for lesions rated with high PIRADS grade5. An important limitation in the present study is that negative patients still might suffer from prostate cancer despite being negative in histopathology. In the future, additional methods such as nuclear medicine might improve the diagnostic performance. Nevertheless, ARTEMIS MRI-ultrasound fusion guided prostate biopsy increases clinical significant prostate cancer detection rate and may improve patient care within the diagnostic concept, although long-term data investigating oncologic outcomes are currently lacking.Acknowledgements
No acknowledgement found.References
1. Sonn, G.A. et al. Value of Targeted Prostate Biopsy Using Magnetic Resonance–Ultrasound Fusion in Men with Prior Negative Biopsy and Elevated Prostate-specific Antigen. European Urology 65, 809-815 (2014).
2. Moore, C.M. et al. Image-Guided Prostate Biopsy Using Magnetic Resonance Imaging–Derived Targets: A Systematic Review. European Urology 63, 125-140 (2013).
3. Marks, L., Young, S. & Natarajan, S. MRI–ultrasound fusion for guidance of targeted prostate biopsy. Current opinion in urology 23, 43-50 (2013).
4.Siddiqui, M.M. et al. Magnetic Resonance Imaging/Ultrasound–Fusion Biopsy Significantly Upgrades Prostate Cancer Versus Systematic 12-core Transrectal Ultrasound Biopsy. European Urology 64, 713-719 (2013).
5. Le, J.D., Huang, J. & Marks, L.S. Targeted prostate biopsy: value of multiparametric magnetic resonance imaging in detection of localized cancer. Asian Journal of Andrology 16, 522-529 (2014).
Figures