4769
Validation of dMRI-Derived Fiber Orientations Using PLI in Human Fetal Hearts1Department of Computer Science, School of Computer and Information Technology, Beijing Jiaotong University, Beijing, People's Republic of China, 2Univ.Lyon, INSA-Lyon, Université Claude Bernard Lyon 1, UJM-Saint Etienne, CNRS, Inserm, CREATIS UMR 5220, U1206, F-69621, LYON, France, Villeurbanne, France, 3Equipe DYCTIM, Laboratoire TIMC-IMAG, UMR5525 CNRS, Université Grenoble Alpes, La Tronche, France, France, 4Jean-Monnet University, Saint-Etienne, France, Saint-Etienne, France
Synopsis
To validate to what extent the fiber orientations derived from diffusion MRI (dMRI) reveal anatomical reality, polarized light imaging (PLI) allowing the fiber orientations of the human heart to be physically measured with high spatial resolution was used. The dMRI and PLI orientation measurements of the same hearts are then compared using a multimodal registration-based framework. Experimental results show that dMRI and PLI have similar variation patterns of elevation or azimuth angles, except that dMRI introduced a decrease of about 24º in transmural elevation angle range. No significant differences were observed on azimuth angle in both modalities.
Introduction
The structure of myocardial fibers plays a fundamental role in the heart’s function and its change was shown to be directly linked to heart diseases (1). To study the myocardial fiber structure in a whole human heart, three main techniques has been reported in previous literatures: histological technique (2), diffusion magnetic Resonance Imaging (dMRI) (3) and Polarized Light Imaging (PLI) (4). PLI is currently considered as the ground-truth of the myocardial cell architecture of ex vivo human hearts owing to its high spatial resolution (90×90×500μm3) and three-dimensional nature from imaging an entire human heart. In contrast, dMRI, which can be used for both ex vivo and in vivo hearts, is the most promising imaging technique for the routine clinical examination of human myocardial fiber structure. However, dMRI does not measure directly the orientations of myocardial fibers, and to what extent the fiber structure obtained from dMRI reveals the anatomical reality has not been quantitatively validated. In this study, we perform quantitative comparisons on fiber elevation and azimuth angles by using PLI data as the ground-truth.Materials and Methods
Nine ex vivo human fetal hearts were first selected for dMRI acquisition on a 3T clinical scanner (MAGNETOM Verio, Siemens AG, Healthcare Sector, Erlangen, Germany) with the spatial resolution of 1.38×1.38×2mm3 and then for PLI acquisition which is destructive and non-backward. The acquired DTI data were in terms of b0 images and DW images of 32 or 192 directions, while the principal direction of myosin filaments from PLI was obtained in the form of azimuth and elevation angle maps with the spatial resolution of 90×90×500 μm3.
Since the original dMRI and PLI data do not have the same spatial resolution and size, it is necessary to register them in order to perform comparisons at the same positions. Our proposed registration-based dMRI validation by PLI ground-truth consists of the following 3 steps: i) Multimodal registration including affine and nonrigid registrations was achieved on elevation angle volumes to register dMRI myocardium to ground-truth PLI myocardium, ii) Transformations including affine and nonrigid registrations were applied to diffusion tensor fields from dMRI data, based on which the registered elevation and azimuth angle volumes were obtained, iii) Normalization of the myocardium was performed in a similar manner to that in (5) to compare the hearts from the two imaging modalities in the same reference coordinates. Quantitative comparisons were carried out on both elevation angles and azimuth angles. Three types of transmural positions are chosen: epicardium of the free wall or right endocardium (Epi) of the septum, endocardium (Endo) of the left ventricle (LV) and zero-crossing contour (Zero-crossing). Zero-crossing contour is formed of the points at which the elevation angle changes sign from positive to negative or contrariwise in the radial direction of myocardium.
Results
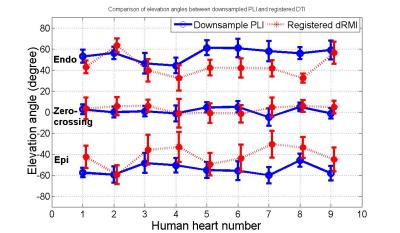
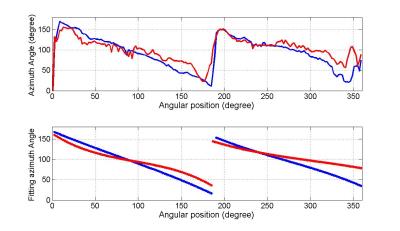
Quantitative comparisons have been performed on both elevation angle and azimuth angle. Fig.1 shows elevation angles along the transmural position in equatorial slices of the nine human fetal hearts. Three types of transmural positions are chosen: epicardium of the free wall or right endocardium of the septum (Epi), endocardium of the LV (Endo) and zero-crossing contour (Zero-crossing). Zero-crossing contour is formed of the points at which the elevation angle changes sign from positive to negative or contrariwise in the radial direction of myocardium. It is observed that the transmural elevation angle at Endo decreased about 11.32º±9.44º, while it increased at Epi about 12.98º±8.08º. That is, the dMRI induces a decrease about 24.31º±14.78º on the transmural elevation angle range. In Fig.2 are plotted the profiles of azimuth angles along the zero-crossing contour and the corresponding 3th-order Legendre polynomial piecewise fitting for downsampled PLI and registered dMRI. It is seen that azimuth angle values from 180° to 0° are periodically visited twice clearly in the LV myocardium for both downsampled PLI and registered dMRI, but the latter has a noisier profile then the former. The 3th-order Legendre polynomial piecewise fitting generates two linear-like descending curves with the visiting range from 160° to 20° for both modalities, but the registered dMRI has less obvious second visiting. Similar comparison results on azimuth angles are observed in the nine hearts.Conclusion
Similar variation pattern on elevation and azimuth angles were found from both dMRI and PLI modalities. This enforces dMRI as a valid imaging technique to characterize fiber orientations of the human heart. However, dMRI introduced a decrease of about 24º in transmural elevation angle range. No significant differences were observed on azimuth angle in both modalities.Acknowledgements
No acknowledgement found.References
1. Weber KT. Cardiac interstitium in health and disease: The fibrillar collagen network. Journal of the American College of Cardiology 1989;13:1637–1652. doi: 10.1016/0735-1097(89)90360-4. 2. D. D. Streeter. Gross morphology and geometry of the heart. In: The Cardiovascular System. Ed.: Handb. Bathesda, B.: American Physiology Society; 1979. pp. 61–112. 3. Basser PJ, Pierpaoli C. Microstructural and physiological features of tissues elucidated by quantitative-diffusion-tensor MRI. 1996. Journal of magnetic resonance (San Diego, Calif.?: 1997) [Internet] 2011;213:560–70. doi: 10.1016/j.jmr.2011.09.022. 4. Jouk PS, Mourad A, Milisic V, Michalowicz G, Raoult A, Caillerie D, Usson Y. Analysis of the fiber architecture of the heart by quantitative polarized light microscopy. Accuracy, limitations and contribution to the study of the fiber architecture of the ventricles during fetal and neonatal life. European journal of cardio-thoracic surgery?: official journal of the European Association for Cardio-thoracic Surgery 2007;31:915–921. doi: 10.1016/j.ejcts.2006.12.040. 5. Geerts L, Bovendeerd P, Nicolay K, Arts T. Characterization of the normal cardiac myofiber field in goat measured with MR-diffusion tensor imaging. Am J Physiol Heart Circ Physiol 2002;283:H139-145. doi: 10.1152/ajpheart.00968.2001.Figures