4764
A Novel 4D Semi-automatic Segmentation Algorithm for Whole-heart 3D Cine Magnetic Resonance Imaging1Massachusetts Institute of Technology, Cambridge, MA, United States, 2Boston Children's Hospital, Boston, MA, United States
Synopsis
Three-dimensional (3D) time-resolved (cine) whole-heart magnetic resonance imaging promises to greatly facilitate comprehensive and evaluation of cardiac function and morphology. We present here a robust semi-automatic 4D segmentation algorithm, using patch-based volumetric segmentation and a temporal image registration method, to reduce the segmentation time of 3D cine datasets to less than 30 minutes and to enable wide clinical use. Resulting volumetric measurements of the left ventricle and right ventricle are aligned with measurements from the current clinical routine. By visualizing the anatomy and dynamics of the heart, we show that 3D cine datasets promise to enhance surgical planning for patients with complex congenital heart disease.
Purpose
Three-dimensional (3D) time-resolved (cine) whole-heart magnetic resonance imaging promises to greatly facilitate comprehensive and evaluation of cardiac function and morphology1. A recently developed free-breathing electrocardiogram and respiratory navigator-gated 2D cine steady-state free precession (SSFP) sequence has shown promise for the assessment of the ventricular volumes and ejection fractions2. However, 2D acquisitions suffer from a coarse through-plane resolution, which limits the fidelity of volumetric measurements and precludes detailed visualization of cardiac structure and function. High resolution 3D cine SSFP promises to enable such visualizations and to provide more accurate measurements of ventricular volumes, which requires ventricular segmentation in multiple high resolution 3D volumes. Manual segmentation of multiple 3D volumes is time intensive (i.e., 4-8 hours of work for a single 3D volume) and therefore clinically impractical. We aim to develop a robust semi-automatic 4D segmentation algorithm to reduce the segmentation time of 3D cine datasets to less than 30 minutes and to enable wide clinical use.
Materials and Methods
Five patients (4 male, age 17.8$$$\pm$$$5.8 years) with complex congenital heart disease were recruited and consented. 3D cine SSFP image series were acquired from each patient on a 1.5T MR scanner (Philips Achieva D-stream) with anterior coil and the following imaging parameters: voxel size ~2 mm3, TR/TE 3.4/1.7 ms, flip angle 60$$$^{\circ}$$$, 30 cardiac phases, and SENSE 3. Scan time was around 10 minutes. To compensate for respiratory-induced heart motion, the reconstruction was performed only using the data acquired within a narrow 5 mm acceptance window around end-expiration. As a routine clinical protocol, 11-13 short-axis 2D cine images were also acquired from each patient with the following imaging parameters: voxel size ~1.8 mm3, slice thickness 8 mm, TR/TE 2.8/1.4 ms, flip angle 60$$$^{\circ}$$$, 30 cardiac phases, and SENSE 2. The 3D and 2D cine SSFP datasets were reconstructed online and analyzed retrospectively for segmentation. Following the current clinical practice, the 2D cine datasets were manually segmented only at the end-diastolic and end-systolic cardiac phases by an expert clinician. The 3D cine datasets were segmented using our proposed semi-automatic algorithm. Using our approach, only 5-10 short-axis "reference" slices (out of 50-100) in the end-diastolic 3D volume are manually segmented, which can be done in less than 30 minutes. The left ventricle (LV) and right ventricle (RV) labels are automatically propagated through the remaining 3D image using a patch-based segmentation algorithm3. Each short-axis slice is segmented using the two closest reference slices. For each patch to be segmented, the ten most similar reference patches are retrieved, where similarity between two patches depends on their intensities, intensity gradients, and physical locations in the image. These ten reference patches contribute estimates of the segmentation for the input patch. Manual editing is then performed to draw the boundary of the two ventricles. Finally, the segmentation of the single 3D volume is propagated to the other 29 volumes in the cine dataset using a temporal image registration method4. The deformation from the manually segmented volume (the template) to the other volumes is estimated while encouraging temporal smoothness within the series. The end-diastolic volume (EDV) and end-systolic volume (ESV) for the left and right ventricles were estimated from the segmentations and compared between the two imaging approaches (2D vs. 3D) using the correlation coefficient. Note that the estimation using our proposed algorithm was blinded to the measurements from the 2D slices.
Results
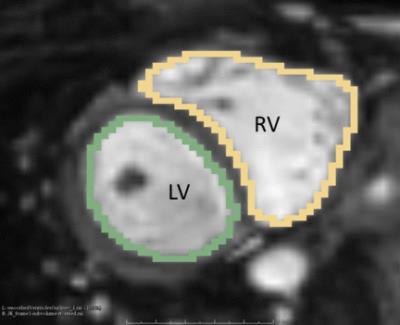
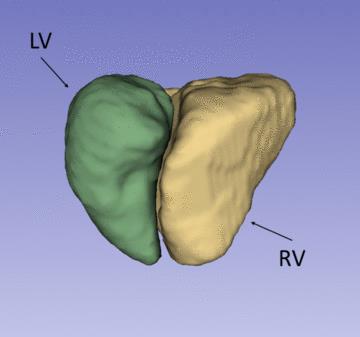
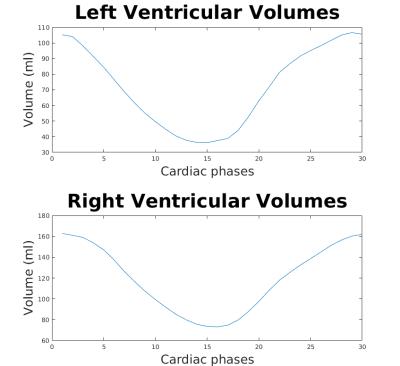
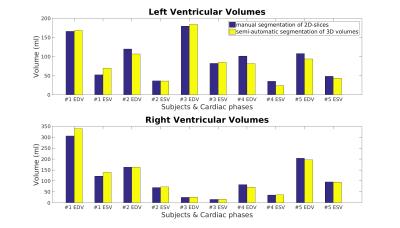
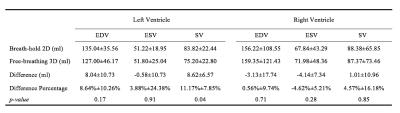
Figure 1 shows a representative dynamical short-axis view of a 3D cine dataset in which LV and RV borders were delineated in different cardiac phases using the semi-automated segmentation algorithm. Figure 2 displays dynamical 3D models of both ventricles generated from the entire image series. The volumetric changes of LV and RV over the cardiac cycles are shown in Figure 3. No significant differences were detected between volumetric measurements except for LV stroke volume from manually segmented 2D images and semi-automatically segmented 3D cine datasets (Figures 4 and 5).
Conclusions
We demonstrated an efficient approach for semi-automatic segmentation of 3D cine SSFP images. Beyond ventricular volume measurements, the method generates 4D heart models for dynamic visualization of ventricle function. Here only left and right ventricles are considered for segmentation. Future work will include segmentation of left and right atrium as well as great vessels from the 3D cine datasets. The 3D cine datasets promise to provide accurate clinical measurements and enhance surgical planning for patients with complex congenital heart disease by visualizing anatomy and dynamics of the heart.
Acknowledgements
We acknowledge support from NIH NIBIB NAC P41EB015902, NIH NICHD U01HD087211, NIH NIBIB R01EB017337, Wistron Corporation, and Siebel Fellowship.References
1. Makowski MR et al. Congenital heart disease: cardiovascular MR imaging by using an intravascular blood pool contrast agent. Radiology 260.3 (2011): 680-688.
2. Moghari MH et al. Free-breathing steady-state free precession cine cardiac magnetic resonance with respiratory navigator gating. Magnetic resonance in medicine 73.4 (2015): 1555-1561.
3. Pace DF et al. Interactive Whole-Heart Segmentation in Congenital Heart Disease. International Conference on Medical Image Computing and Computer-Assisted Intervention. Springer International Publishing, 2015.
4. Liao R et al. Temporal Registration in In-Utero Volumetric MRI Time Series. International Conference on Medical Image Computing and Computer-Assisted Intervention. Springer International Publishing, 2016.
Figures