4753
Slice Realignment for Motion-Corrupted Stacks of Short-Axis Cine Cardiac MR Images based on 3D Probabilistic Edge Maps1Imperial College London, London, United Kingdom
Synopsis
Short-axis cine cardiac MR image stacks are acquired during multiple breath-holds, which often causes a misalignment of several slices. We propose a technique for in-plane spatial realignment of motion-corrupted short-axis slices which uses probabilistic edge maps of the myocardium (generated with decision forests) as input to image registration. The proposed technique was quantitatively tested on a dataset of motion-free stacks artificially corrupted by in-plane motion. Overlap measures such as the Dice coefficient - computed on myocardial masks segmented respectively on motion-free, motion-corrupted and motion-corrected stacks - suggest that the proposed technique is able to correctly compensate for slice misalignment.
Purpose
The cardiac MR sequence most commonly used in the clinical practice is arguably the short-axis (SA) SSFP cine, which consists of 10-14 parallel slices at 20-30 frames per cardiac cycle acquired during multiple subsequent breath-holds. Although subjects are asked to hold their breath always at the same breath-hold position, this is often difficult to achieve. Differences between breath-hold positions determine an apparent misalignment of the heart in the different slices, which can introduce errors in the subsequent analyses and visualizations1. Accordingly, we propose a technique for in-plane spatial realignment of potentially motion-corrupted SA stacks.Methods
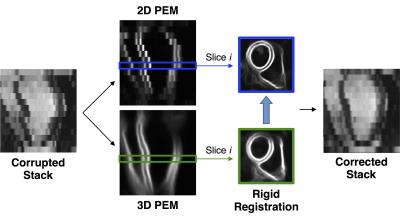
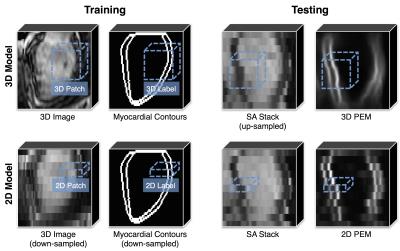
The proposed technique (with main steps reported in figure 1) makes use of probabilistic edge maps (PEMs) generated using structured decision forests2. Through training, structured decision forests can associate each 2D or 3D patch (extracted from the SA stack) with a label consisting in the contours of the myocardium in that patch. During testing, labels extracted from neighboring patches are averaged in order to produce the final PEM. In the proposed technique, both a 3D and a 2D PEM models are trained. For the training of the 3D model, we used a dataset of 359 3D images (acquired during a single breath-hold, thus motion-free) together with myocardial contours, with a patch thickness equal to that of 5 SA slices combined (figure 2, upper left). As a consequence, at testing time, the 3D model is able to associate a potentially motion-corrupted stack with a virtually motion-free 3D PEM (figure 2, upper right). For the training of the 2D model, we down-sampled the same 3D dataset along the z direction of a factor of 5 (in order to match the slice thickness of a common SA stack), and used 2D patches (figure 2, lower left). At testing time, the 2D model associates each slice with an independent 2D PEM, thus preserving the potential misalignment between slices (figure 2, lower right). The final step of the technique consists in the slice-by-slice rigid registration (performed using sum of squared differences as similarity metric and only allowing in-plane rotations and translations) between 3D and 2D PEMs: the estimated transformation is then applied to each slice of the stack, thus performing the realignment.
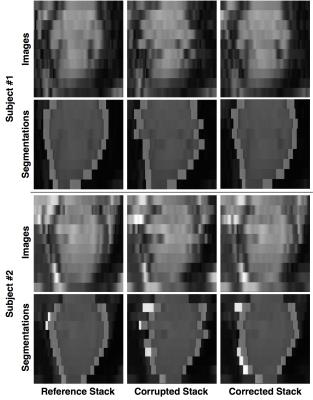
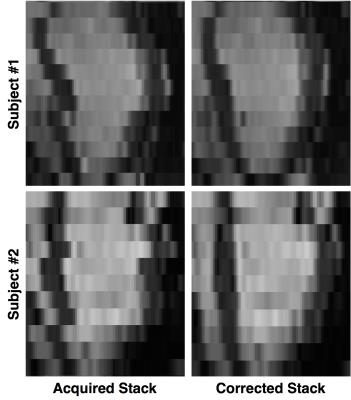
The proposed approach was tested on a separate dataset of 24 motion-free 3D images. The images were first down-sampled in the z direction to mimic SA stacks. Then, the slices were artificially corrupted by applying random in-plane rigid transformations to simulate the effects of different breath-holding positions (probability of slice corruption=0.6, range of translations along the x-axis=±4.5mm, range of translations along the y-axis=±6.5mm, range of rotations=±5.5 degrees). The technique was then applied to perform slice realignment. MR acquisition protocol details are TR/TE=3.0/1.5ms, flip angle=50 degrees, reconstructed voxel size=1.2×1.2×2mm, number of slices=50-60, cardiac phases=20, sensitivity encoding (SENSE) factor=2.0. To assess the effectiveness of the proposed approach, a 3D multi-atlas segmentation technique3 was applied to the SA stacks before motion-corruption (reference), after motion-corruption and after motion-correction. The Dice coefficient for the LV myocardial mask (defined as the volume between the epicardial and the endocardial boundaries) was computed for each stack between reference and after corruption (DC_pre), and between reference and after correction (DC_post), respectively. Finally, the technique was also applied to a dataset of 16 normally acquired SA stacks (reconstructed voxel size=1.2×1.2×10mm) to qualitatively assess its accuracy.
Results
The average Dice coefficient for the LV myocardial mask was considerably improved by the application of the proposed technique: DC_pre=0.72±0.09, DC_post=0.81±0.04 (paired t-test, p<10-5). Qualitative results for both the artificially corrupted stacks and the acquired ones are reported in figures 3 and 4, respectively.Discussion
The reported results indicate that the presented technique is able to significantly reduce slice misalignment. Of note, however, the proposed approach is limited to in-plane realignment; moreover, the correction of stacks with 5 subsequent misaligned slices is likely to fail due to the upper bound determined by the size of the 3D patches. Differently from our previous work4, in the present implementation we perform the slice-by-slice registration between 3D and 2D PEMs instead of the registration between 2D PEMs of adjacent slices, thus avoiding error propagation through the stack.Conclusion
We presented a technique for in-plane spatial realignment of motion-corrupted SA slices based on probabilistic edge maps generated with decision forests, and we tested it on artificially corrupted SA stacks in order to assess its effectiveness. The results suggest that the technique is able to effectively compensate for slice misalignment, and could thus be adopted as a preprocessing step for SA stacks before running subsequent analyses.Acknowledgements
The main author benefits from a Marie Sklodowska-Curie european Fellowship.References
1. Chandler AG, Pinder RJ, Netsch T, Schnabel JA, Hawkes DJ, Hill DLG, Razavi R. Correction of misaligned slices in multi-slice cardiovascular magnetic resonance using slice- to-volume registration. Journal of cardiovascular magnetic resonance 2008;10:13.
2. Dollar P, Zitnick CL. Fast Edge Detection Using Structured Forests. IEEE Transactions on Pattern Analysis and Machine Intelligence 2015;37(8):1558–1570.
3. Bai W, Shi W, O’Regan DP, Tong T, Wang H, Jamil-Copley S, Peters NS, Rueckert D. A probabilistic patch-based label fusion model for multi-atlas segmentation with registration refinement: Application to cardiac MR images. IEEE Trans- actions on Medical Imaging 2013;32(7):1302–1315.
4. Oktay O, Tarroni G, Bai W, de Marvao A, O’Regan D, Cook S, Rueckert D. Respiratory Motion Correction for 2D Cine Cardiac MR Images using Probabilistic Edge Maps. Computing in Cardiology 2016, in press.
Figures