4745
Using Hyperpolarized 129Xe in Human Participants to Perform Functional Magnetic Resonance Imaging (fMRI)Francis Hane1, Tao Li1, Jane M Lawrence-Dewar2, Ayman Hassan3, Karl Granberg3, Raiili Pellizzari1, Jennifer Anne Plata4, and Mitchell Albert1,5
1Lakehead University, Thunder Bay, ON, Canada, 2Thunder Bay Regional Research Institute, Thunder Bay, ON, Canada, 3Thunder Bay Regional Health Sciences Centre, 4Lakehead University, 5Northern Ontario School of Medicine, ON, Canada
Synopsis
We demonstrate the use of hyperpolarized (HP) 129Xe MRI as a novel fMRI modality. We successfully obtained axial HP Xe fMRI maps from healthy humans throughout the conduct of a 1-back memory task. Our preliminary results suggest that HP 129Xe fMRI may have a sensitivity of up to an order of magnitude more than BOLD fMRI.
Audience & Purpose
Hyperpolarized (HP) xenon-129 (129Xe) can be used as a contrast agent to image such diverse phenomenon as lung pathologies and brain perfusion 1, 2. Localized brain perfusion has been directly correlated to neuronal activity 3. Because HP 129Xe is detectible within the cerebro-vasculature 1, it has the potential to spatially localize areas of brain activity 2 in a method similar to BOLD fMRI 4. In this work, we present the preliminary results (n=6) of what we believe to be the first HP 129Xe fMRI maps of human participants. Our preliminary results suggest that 129Xe fMRI can achieve a signal enhancement of up to 20% potentially making HP 129Xe an order of magnitude more sensitive than BOLD fMRI.Methods
All procedures were reviewed and approved by local institutional Research Ethics Boards and all participants provided written informed consent. Brain imaging was performed in five healthy volunteers (male & female, ages 24-64) using a 3TPhilips Achieva MRI, equipped with a 129Xe/1H dual tuned head-coil (Clinical MR Solutions, Brookfield, WI). Enriched (84%) 129Xe gas was polarized to 26-30% using a Xemed polarizer (Xemed LLC, Durham). Each participant inhaled 1 L of HP 129Xe and held their breath for 20 s. Three dynamic Xe images were acquired using a 2D fast field echo (FFE) technique: the first at 10 s following inhalation, the second at the end of the breath hold, and the third at 10 s following Xe exhalation. Imaging parameters were as follows: FOV = 250 mm x 250 mm, matrix = 32x32, TR/TE = 250 ms/0.84 ms, NSA = 1, FA = 12°, Bandwidth = 150 Hz/pixel. Later, the participant was asked to conduct a 1-back task 5 whereby the participant was presented with a series of letters. If two subsequent letters were identical, the participant had to respond by pressing a button. 129Xe MR imaging during the stimulus/task was conducted identically to the baseline image. 1H MRI were acquired using a turbo spin echo sequence utilizing the following parameters: FOV = 250 mm x 250 mm, matrix = 256x256, TR/TE = 3 s/80 ms, NSA = 2, FA = 90°. 129Xe MRI were processed using a custom Matlab (MathWorks, Natick, MA, USA) script. Xenon images were zero-filled to 256x256, registered to the center of the field of view. All participants' images were averaged (n=12, 2 scans/participant) to construct the images shown in Fig. 1. The baseline 129Xe MR image was subtracted from the stimulus/task 129Xe MR image to create a 129Xe fMRI map. The 129Xe fMRI map was overlaid on a 1H MRI of the brain.Results and Discussion
There were substantial differences in signal intensity between the baseline and 1-back task scans. The first dynamic scan, acquired 10 s into the breath hold showed a slightly lower Xe perfusion throughout the cerebrum in the task scan compared to the baseline scan. However, at the conclusion of the breath-hold, the Xe signal throughout the cerebrum was 50%-100% greater in the task scan. Ten seconds following the conclusion of the breath hold, we observed a ca. 20% greater signal in the posterior of the brain, in the area of the visual cortex for the task scan and 50% lower signal in the anterior region of the brain perhaps in the location of the prefrontal cortex. HP Xe fMRI operates primarily on the assumption that stimulated neurons are correlated with increased perfusion. Therefore, an increase in Xe to stimulated brain regions results 3 in a localized Xe signal enhancement. Secondarily, an increased concentration of oxyhemoglobin in stimulated regions results in a longer T1 of HP Xe and therefore greater Xe signal in stimulated regions. We hypothesize that the broad stimulus and subject response involved in the 1-back task activates the visual, working memory, and motor regions of the brain throughout the breath hold period. The signal enhancement observed was an order of magnitude greater than observed in typical proton BOLD fMRI studies 3. Future studies are needed to refine this technique, both for acquisition and analysis, which will allow more precise localization of the Xe signal changes, and further elucidation of the mechanism involved.Conclusions
To our knowledge, this is the first demonstration of a signal enhancement observed in the human brain during a functional stimulus, detected using hyperpolarized 129Xe MRI, in a manner similar to that of fMRI. These preliminary results suggest that 129Xe can be used as a highly sensitive fMRI modality, which may lead to greater insights in the understanding of brain function.Acknowledgements
This work is supported by a Weston Brain Institute grant to MA and postdoctoral fellowships to FH from the BrightFocus Foundation and the Canadian Institutes for Health Research (CIHR). MA wishes to acknowledge the Weston Brain Institute and its generous donors for support of this work. FH wishes to acknowledge the BrightFocus Foundation and its generous donors for support of this work.References
1. Rao M, Stewart NJ, Norquay G, Griffiths PD, Wild JM: High resolution spectroscopy and chemical shift imaging of hyperpolarized 129 Xe dissolved in the human brain in vivo at 1.5 tesla. Magn Reson Med 2016; 75:2227–2234. 2. Mazzanti ML, Walvick RP, Zhou X, et al.: Distribution of Hyperpolarized Xenon in the Brain Following Sensory Stimulation: Preliminary MRI Findings. PLoS One 2011; 6:e21607. 3. Logothetis NK, Pauls J, Augath M, Trinath T, Oeltermann A: Neurophysiological investigation of the basis of the fMRI signal. Nature 2001; 412:150–157. 4. Ogawa S, Lee T, Nayak A, Glynn P: Oxygenation-sensitive contrast in magnetic resonance image of rodent brain at high magnetic fields. Magn Reson Med 1990; 6:68–78. 5. Kirchner WK: Age differences in short-term retention of rapidly changing information. J Exp Psychol 1958; 55:352–8.Figures
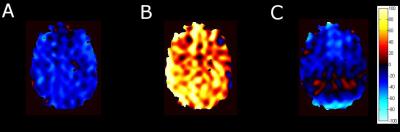
Signal averaged HP Xe fMRI maps in the axial plane of the brains of five healthy humans doing a
“1-back” test. fMRI maps were acquired 10 seconds into the Xe breath hold (A),
at the end of the breath hold (B), and 10 seconds following the breath hold
(C). fMRI maps were acquired by subtracting the baseline image from the stimulus/task
image. In
(C), signal enhancement appears to originate from the posterior region of the
brain. ‘Cold” shades indicate a greater signal for the baseline than the task
whereas “hot” shades indicate greater signal from the task scan.
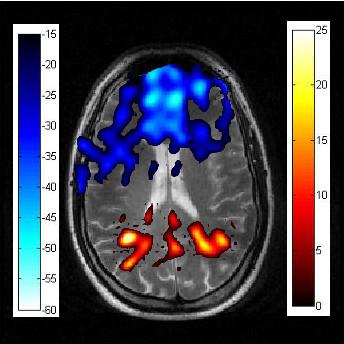
Signal averaged fMRI map of five
healthy humans conducting a “1-back” task following a Xe breath hold. Xe fMRI
is overlaid on a 1H MRI in the axial plane. Signal from the 1-back task appears
to involve activation of the posterior regions of the brain. ‘Cold” shades
indicate a greater signal for the baseline than the task whereas “hot” shades
indicate greater signal from the task scan than the baseline scan. The color bar denotes percent increase (hot) or decrease (cold) with reference to the baseline scan.