4744
Non-Invasive Cerebrovascular Pulsatility Measurement via Cardiac Sorting of BOLD Data: An Investigative Study Using Exercise-Induced Hypotension in Adolescents1University of Toronto, Toronto, ON, Canada, 2Sunnybrook Research Institute, Toronto, ON, Canada, 3Heart & Stroke Foundation Canadian Partnership for Stroke Recovery, Toronto, ON, Canada
Synopsis
Arterial pulsatility increases with age and is linked to small vessel damage and neurodegeneration. Our group has developed a method that fits a pulsatility model to BOLD temporal volumes based on their cardiac cycle position. We test this method in a healthy adolescent group before and after a physiological stressor, i.e. 20 minutes of moderate intensity exercise. Brain pulsatility was significantly lower in BOLD scans taken 20 minutes after exercise cessation, supporting the viability of this method to track brain arterial stiffness non-invasively.
Purpose
Blood leaves the heart in a pulsatile manner and is dampened by elastic properties of arteries prior to reaching the microcirculation. Arterial elasticity is highest in young people, while age-related stiffening is thought to contribute to target organ tissue damage1. Elevated pulsatility in the brain has been implicated in the progression of white matter hyperintensities, cognitive impairment and the development of neurodegenerative diseases2, 3. Thus, characterizing brain pulsatility may have broad clinical and theoretical implications. Unfortunately, the presence of pathology typically adds confounds, making it difficult to validate results using novel methods; therefore we test our method of extracting tissue-level cerebrovascular pulsatility in a healthy young cohort.
In the current study, we developed a non-invasive method based on blood oxygenation level dependent (BOLD) contrast images. Although BOLD is used to probe neurovascular signals, it is also influenced by non-neuronal sources, such as the influence of respiratory and cardiac cycles4. We evaluated the efficacy of this new brain pulsatility method by testing for the effect of post-exercise hypotension; hypothesizing that brain pulsatility will be reduced post-exercise5.
Methods
BOLD data were collected at 3T (Philips Achieva), with 500 Hz cardiac sampling via a pulse oximeter in a cohort of adolescent participants (N=44, 24 female, age: 16 ± 2 years). We used AFNI’s RETROICOR post-processing software to remove respiratory influences. Participants underwent two BOLD scans (resting state and task) prior to, and approximately 20 minutes following, 20 minutes of moderate intensity (achieving ~70% of their maximum heart rate) aerobic cycling (i.e. 4 BOLD scans per participant).
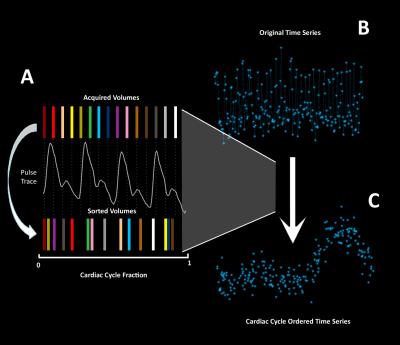
After RETROICOR, motion correction, slice timing correction and spatial smoothing, BOLD temporal volumes were re-sorted (Figure 1) based on their location in the cardiac cycle (bin width = 10 ms); voxel-wise pulsatile features were ascertained by fitting a seven-term Fourier series and comparing this fit to 45000 null BOLD cardiac traces generated by random permutation of the BOLD data across the cardiac cycle. Voxels were considered significant when their R2 value exceeded 5 standard deviations of the null fits, corresponding to a non-parametric p<0.001.
Results
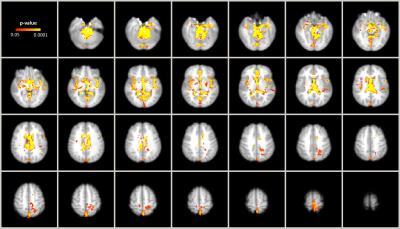
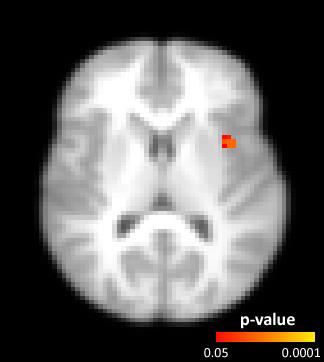
The (mean±std) percentage of intracranial voxels showing a brain pulsatility pattern was 11.1±4.2% before and 9.4±4.0% after exercise. Post-exercise resting state scans were found to have significantly lower pulsatility (p<0.05) than at baseline, in areas that tend to contain large arteries, ventricles, the superior sagittal sinus, insula, and precentral gyrus, (Figure 2) when compared using non-parametric group analysis. In contrast, only a portion of the left middle cerebral artery region, within proximity of the insula, showed decreased pulsatility features within the task BOLD scans after exercise (Figure 3).Discussion
We demonstrate that it is feasible to re-sort BOLD temporal volumes based on their expected location in the cardiac cycle. Our approach used a rigorous non-parametric method and extends from previous cardiac-brain BOLD data in the literature4. In particular we found cardiac-related BOLD sources produce tractable effects on the brain that depends on the brain region (i.e. proximity to a larger artery) and physiology (i.e. after acute exercise blood pressure decreases). Task-BOLD data showed a less dramatic brain pulsatility effect, due in large part to our experimental design (i.e. resting state BOLD always preceded task BOLD). In a healthy young cohort, we expect physiological recovery after exercise to occur at the time span that corresponded to the follow-up task-BOLD scan.Conclusion
The cardiac-cycle BOLD re-sorting method is capable of detecting changes in pulsatility within the brain after exercise, an effect that appears to be time-dependent over the course of exercise recovery. Additionally, while the predominate, observed changes are within the large vessels, changes in other regions were also detectable with our method. Future work will characterize pulsatility in populations with neurological diseases to determine whether this method is useful to quantify neurovascular risk.Acknowledgements
No acknowledgement found.References
1. O'Rourke, MF and Hashimoto, J. Mechanical Factors in Arterial Aging: A Clinical Perspective. JACC. 2007; 50(1): 1-13
2. Makedonov, I et al. BOLD fMRI in the White Matter as a Marker of Aging and Small Vessel Disease. PLOS One. 2013; 8(7): 1-9
3. Webb, AJ et al. Increased cerebral arterial pulsatility in patients with leukoaraiosis: arterial stiffness enhances transmission of aortic pulsatility. Stroke. 2012; 43(10): 2631-2636
4. Dagli, MS et al. Localization of Cardiac-Induced Signal Change in fMRI. NeuroImage. 1999; 9: 407-415
5. Chen, CY and Bonham, AC. Postexercise Hypotension: Central Mechanisms. Exerc Sport Sci Rev. 2010 Jul; 38(3): 122-127
Figures