4732
Improving Multiband EPI pCASL Imaging with Dynamic Frequency FeedbackDingxin Wang1,2, Gregory Metzger2, Kamil Ugurbil2, and Xiufeng Li2
1Siemems Healthcare, Minneapolis, MN, United States, 2Center for Magnetic Resonance Research-Radiology, University of Minnesota, Minneapolis, MN, United States
Synopsis
Dynamic frequency feedback to multiband (MB) EPI PCASL excitation, fat saturation, and labeling RF pulse frequency can improve spatial and temporal perfusion signal-to-noise ratio (sSNR and tSNR) and cerebral blood flow (CBF) measurement reproducibility of MB-EPI PCASL. Dynamic frequency feedback helps maintain optimal labeling efficiency, achieve stable fat saturation, and correct scanner drift during MB-EPI PCASL measurements.
Purpose
Multi-band (MB) echo planar imaging (EPI) for
pseudo-continuous arterial spin labeling (PCASL) imaging has been
recently developed1,2. Particularly, high-resolution whole brain
MB-EPI PCASL imaging study indicates that MB-EPI PCASL provides superior spatial and temporal perfusion signal-to-noise ratio
(sSNR and tSNR) efficiencies and improves
the accuracy of cerebral blood flow (CBF) quantification over
regular EPI-PCASL3. However, dynamic B0 changes, caused by
respiration and scanner frequency drift, leads to dynamic EPI image artifacts, such as pixel intensity changes and pixel location shifts in
phase-encoding direction. Furthermore, dynamic frequency shift may potentially
introduce variations in fat saturation and reduce labeling efficiency of PCASL.
Due to simultaneous multi-slice (SMS) acquisition and increased scanner duty
cycle of MB-EPI, these dynamic B0-induced artifacts can be more severed on
MB-EPI PCASL with a high MB factor, which will negatively impact sSNR, tSNR, and perfusion quantification of MB-EPI PCASL. Dynamic B0 changes can be detected and corrected using
navigator signals4 and methods such as correction of the dynamic
off-resonance in k-space5. Real-time dynamic frequency correction
has been demonstrated to improve data quality of single-voxel MR spectroscopy6.
Dynamically updating excitation, fat saturation, and labeling RF pulse frequency
of MB-EPI PCASL using real-time frequency feedback (FB) detected from navigator
signals may help mitigate problems associated with dynamic frequency changes.
In this study, we tested the hypothesis that dynamic frequency feedback can
improve sSNR, tSNR, and CBF measurement reproducibility of MB-EPI PCASL. Methods
With IRB approval and informed consent, MB-EPI PCASL imaging studies in 5 healthy volunteers were performed on a 3T MRI scanner (MAGNETOM Prisma, Siemens Healthcare, Erlangen, Germany) using a 32-channel head coil. Three MB-EPI PCASL protocols were compared: (1) No dynamic frequency feedback (B0 shimming volume covering both imaging and labeling planes) (2) Dynamic frequency feedback (B0 shimming volume covering both imaging and labeling planes) (3) Dynamic frequency feedback (B0 shimming volume covering only image planes). Imaging parameters include 3800 ms/14 ms TR/TE, multiband factor 6, 3.5x3.5x3.5 mm3 resolution, 36 slices, 20% slice gap, 2.0 sec delay time, fat saturation, 40 parried pCASL measurements plus 2 additional M0 measurements. Within each ASL series scan, additional 200 noise images were collected for noise measurement. Perfusion sSNR and tSNR were calculated from each subject. Reproducibility study was performed in 3 subjects. MB-EPI PCASL with and without dynamic frequency feedback were executed twice. Coefficient of variation (CV) between the two CBF maps was calculated. sSNR, tSNR, and CV measurements within grey and white matters between MB-EPI PCASL protocols were compared using paired t-tests with a=0.05.Results
For all MB-EPI PCASL measurements, dynamic frequency feedback with B0 shimming volume covering imaging and labeling planes has the highest sSNR and tSNR. sSNR ratio and tSNR ratio normalized to dynamic frequency feedback with B0 shimming volume covering imaging and labeling planes are shown in Figure 1. For MB-EPI PCASL with B0 shimming volume covering imaging and labeling planes, sSNR is significantly higher in frequency feedback protocol than no frequency feedback protocol (P = 0.015 in grey matters and P < 0.002 in white matters (all n =5)). tSNR also shows significant improvement with dynamic frequency feedback over without frequency feedback when pooling data of grey and white matters together (P = 0.013, n =10), although it is not statistically significant for grey and white matters individually (P = 0.131 in grey matters and P = 0.061 in white matters (all n =5)). For MB-EPI PCASL with dynamic frequency feedback, sSNR and tSNR of measurements with B0 shimming volume covering only image planes are significantly less than those of measurements with B0 shimming volume covering both imaging and labeling planes (sSNR: P < 0.008 in grey matters and P < 0.005 in white matter tSNR: P = 0.038 in grey matters and P = 0.032 in white matters (all n= 5)). The calculated CBF maps with and without frequency feedback from one subject show comparable CBF distributions (Figure. 2). From the reproducibility study of CBF measurement, MB-EPI PCASL with dynamic frequency feedback shows significantly better reproducibility than no frequency feedback, i.e. smaller CV between repeated measurements, when grouping samples of grey and white matters together (5.1% vs 9.1%, P = 0.031, n = 6).Discussions
Our data indicates dynamic frequency feedback
benefits sSNR, tSNR, and CBF measurement reproducibility of MB-EPI PCASL.
Frequency feedback helps maintain optimal labeling efficiency, achieve stable
fat saturation, and correct scanner drift.
Conclusions
Dynamic frequency feedback to multiband (MB) EPI PCASL excitation, fat saturation, and labeling RF pulse frequency can improve sSNR, tSNR, and cerebral blood flow (CBF) measurement reproducibility of MB-EPI PCASL.Acknowledgements
P41 EB015894, P30 ICC and Human Connectome Project (1U54 MH091657), UMF0003900, and UL1TR000114. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health.References
1. Li et al. ISMRM 2014:4557. 2. Li et al. ISMRM 2014: 995. 3. Li et al. Neuroimage 2015. 4. Hu et al. MRM 1994. 5. Pfeuffer et al. MRM 2002. 6. Keating et al. MRM 2012.Figures

Figure 1. sSNR ratio and tSNR ratio normalized to dynamic frequency feedback with B0 shimming volume
covering imaging and labeling planes. FB = frequency feedback with B0 shimming volume covering imaging and labeling planes; nFB = No frequency feedback; FBish = frequency
feedback with
B0 shimming volume covering only imaging planes; GM = grey matter; WM = white
matter
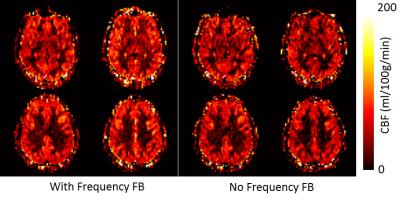
Figure 2. Representative cerebral
blood flow (CBF) maps with
and without dynamic frequency
feedback MB-EPI PCASL from one subject.