4728
Imaging changes in cardiorespiratory pulsation amplitude of the brain during breathold - an MREG-study.1OFNI/Radiology, Oulu University Hospital, Oulu, Finland
Synopsis
Glymphatic pulsation mechanisms clear the brain by using physiological pulsations to drive CSF through the brain tissue. Magnetic resonance encephalography (MREG), an ultra-fast inverse imaging technique, was recently able to map three basic mechanisms driving the glymphatic brain clearance; arterial pulsations, respiratory venous pulses and slow vasomotor waves. In this study we demostrate that the MREG can also detect changes in the amplitudes of the pulsations driving the clearance. The mapping of the physiological pulsation amplitude changes can be used to quantify changes in glymphatic clearing mechanims that precede neurodegeneration.
Introduction:
Recently magnetic resonance ncephalography (MREG) technique was able to physiological pulsations that drive glymphatic clearance mechanisms in the human brain1. Changes in these pulsations amplitudes can alter glymphatic brain clearance, which is known to induce neurodegenerative diseases, such as Alzheimers2. In this study we hypothesize that MREG can reflect measure alterations in cardiovascular and respiratory brain pulsation amplitude induced by breath holding (BH).
Methods:
Eight subjects (aver 25 years, 2 female) were scanned during 5 repetitive 32 sec (1.5 min rest interleaved)
BH-periods after informed consent. Ethical approval was granted by the loca IRB
for using 3T Siemens SKYRA with 32-channel head coil. Imaging was performed
with a single shot MREG sequence, with under-sampled clockwise/anti-clockwise
stacks of spiralsk-space (TR 100 ms, FA 25°)3. Anatomical T1 MPRAGE
0.9 mm cubic was used to align data with MNI template in FSL. A double verified
physiological monitoring was used to obtain cardiac and respiratory pulsation
data (Scanner ECG, resp bellows & GE's Datex-Ohmeda anesthesia monitor SpO2
and ETCO2)4. GroupICA was run to obtain default mode (DMNvmpf &
DMN pcc), CSF ventricles, arterial and sagittal sinus venous MREG signal. After FSL
MELODIC preprocessing (mcflirt, bet, realign, 5 mm FWHM smoothing, 125 s HP
filter), the MREG data was individually band passed for physiological (Resp
0.25-0.4 Hz, Card 0.8-1.2 Hz) frequencies based on verified individual
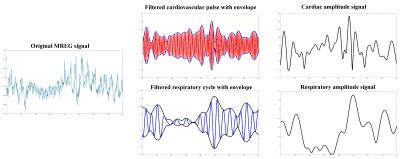
pulsations. The amplitude of the pulsations were calculated from
matlabR2016 envelope function as a difference between the upper peak and lower
thorough envelopes, c.f. Fig 1. Melodic ICA timecourse was obtained for mean
MREG signal of the selected ROI's (DMNvmpf&pcc, Sagital sinus, major
arteries and CSF ventricles).
Results:
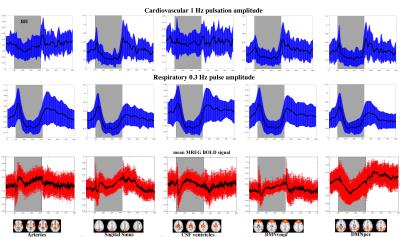
The cardiovascular pulsation amplitudes drop during early BH and overshoot after BH in the sagittal sinus and cortical DMN ROI's. Notably, the arterial and CSF pulsation amplitude changes are smaller in comparison, however also they show increased amplitude after BH. The respiratory pulse amplitude is uniform in all areas; the intial deep inhalation during BH onset increases amplitude which is then followed by marked drop in the breathing amplitude during BH. After BH there is an overshoot during re-breathing. The sagittal vein and the cortical DMN respiratory overshoots after BH are smaller than those in the CSF and arterial areas, which is interestingly reverse to the cardiovascular pulsations.
The averaged MREG signal change reflecting classical BOLD signal below in Fig.2. illustrates an elevation of the MREG signal towards the end of BH in all areas. There is an initial short drop in average signal that turns into signal elevation in the latter half of the BH. There is a marked signal intensity increase in the DMNpcc and especially sagital vein after the BH. The DMNpcc shows interesting prolonged signal drop comapred to all other ROI's.
Conclusion:
Critically
sampled MREG reflects changes in amplitudes of cardiovascular and respiratory
pulsations in the human brain within physiological range during BH.
Interestingly the the arterial amplitude reactions are more marked in the
cortical DMN near the peripheral venous side vs. central arteries and CSF
spaces. Conversely, the respiratory pulsation amplitude rebounce after the BH
in the cortex and veins seems relatively smaller than that in central arteries
and CSF. This suggests somewhat opposing reservoire in these areas and may be
used in differentiating MREG signal source. The sensitivity to physiological range
pulsation amplitude changes may be usable in detecting disease related changes
in mechanisms driving the glymphatic brain clearance.
Acknowledgements
Academy of Finland #275352 & JAES-foundation Grants are cordially acknowledged for supporting the study.References
1. Kiviniemi, V. et al. Ultra-fast magnetic resonance encephalography of physiological brain activity - Glymphatic pulsation mechanisms? J. Cereb. Blood Flow Metab. Off. J. Int. Soc. Cereb. Blood Flow Metab. 36, 1033–1045 (2016).
2. Jessen, N. A., Munk, A. S. F., Lundgaard, I. & Nedergaard, M. The Glymphatic System: A Beginner’s Guide. Neurochem. Res. 40, 2583–2599 (2015).
3. Assländer, J. et al. Single shot whole brain imaging using spherical stack of spirals trajectories. NeuroImage 73, 59–70 (2013).
4. Korhonen, V. et al. Synchronous multiscale neuroimaging environment for critically sampled physiological analysis of brain function: hepta-scan concept. Brain Connect. 4, 677–689 (2014).
Figures