4719
Non-invasive MR Thermometry in a Non-human Primate Model of Acute Ischemic StrokeSeena Dehkharghani1, Candace Fleischer2, Deqiang Qiu3, and Frank Tong4
1Radiology, New York University, New York, NY, United States, 2Radiology and Imaging Sciences, Emory University, Atlanta, GA, United States, 3Radiology, Emory University, Atlanta, GA, United States, 4Radiology, Emory University
Synopsis
Temperature dysregulation is deeply implicated in potentiation of cerebrovascular ischemia. We present a multi-phasic, MR thermographic study in a non-human primate (NHP) model of MCA infarction, hypothesizing detectable brain temperature disturbances and brain-systemic temperature decoupling. Successful physiologic and continuous post-ischemic cerebral MR thermography was conducted, and prescribed in an NHP infarction model to facilitate translatability. The results confirm hypothesized temperature disturbance and decoupling of physiologic brain-systemic temperature gradients. These findings inform a developing paradigm of brain thermoregulation, and the applicability of brain temperature as a neuroimaging biomarker in CNS injury.
INTRODUCTION
Cerebral temperature regulation remains poorly understood and generally under explored by comparison with the closely related physiologic processes of cerebral blood flow and metabolism. Brain temperatures under physiologic conditions are preserved in delicate homeostasis through the interplay of heat production, primarily in the form of metabolic thermal waste, and heat dissipation, driven largely through the heat sink effects of cooler arterial blood. Even modest elevations in systemic temperatures may rapidly potentiate injury to the neurovascular unit, particularly in the aftermath of ischemic insults. Biophysical simulations and limited in vivo studies have indicated the presence of gradients between systemic and brain temperatures, as well as throughout the brain itself, both of which may vary following brain injury. A generalizable paradigm for cerebral temperature dysregulation following acute ischemic stroke (AIS) is challenged by a lack of brain thermometry methodologies unencumbered by prohibitive invasiveness, cost, and constrained characterization of spatial gradients. We recently reported successful optimization of non-invasive cerebral MR thermometry in a non-human primate (NHP) model of AIS. Further improvements in magnetic field shimming and acquisition strategies motivate deeper study of the extent and temporal nature of temperature disturbance in the NHP brain following ischemia. In this study we report multi-phasic thermography of the NHP brain following controlled stroke induction, hypothesizing disturbance in brain temperatures and decoupling of brain-systemic temperature gradients.METHODS
Multi-phasic brain MR thermometry was conducted in three rhesus macaque during: 1) baseline physiologic conditions one week preceding ictus; 2) continuous post-ischemic imaging for seven hours following stroke induction; and 3) delayed post-ischemic (post-stroke day 1) conditions. Fully non-invasive brain thermometry was acquired using a modified multi-voxel spectroscopy approach employing semi-LASER MRSI for detection of the proton resonance frequency chemical shift difference between water and neuronal N-acetylaspartate. The interaction between cerebral and systemic temperatures, and time following stroke induction was characterized using a mixed-effects model.RESULTS
Following MCA infarction, progressive cerebral hyperthermia was detected non-invasively in all three subjects, in all cases significantly exceeding slower rising systemic temperatures. Relative to time, highly significant associations were observed for systemic temperature, as well as regional and global brain temperatures (F-stat p.0005 for all regressions). Significant differences in the relationship between temperature and time following stroke onset were detected when comparing systemic temperatures with ipsilateral (p=.007), contralateral (p=.004), and infarction core (p=.003) temperatures following multiple comparisons correction. Significant associations were observed between infarction volumes and both systemic (p≤.01) and ipsilateral (p=.04) brain temperatures, but not contralateral brain temperature (p=.08).DISCUSSION
Non-invasive brain MR thermometry was conducted under normal physiologic and continuous post-ischemic conditions in a translatable non-human primate model of acute ischemic stroke. Our findings confirmed our hypothesized uncoupling of brain and systemic temperatures following stroke induction, and reaffirm known tendencies towards systemic febrile conditions in large vessel occlusion stroke. We anticipate these findings may offer translational insights into cerebral thermoregulation and may inform future studies of brain temperature as a non-invasive biomarker in cerebrovascular injury.CONCLUSION
Non-invasive brain thermometry is feasible in the acute stroke setting, and brain temperature fluctuations may be observed which cannot be directly derived from systemic febrile responses due to uncoupling of normal baseline brain and systemic temperature gradients.Acknowledgements
No acknowledgement found.References
No reference found.Figures
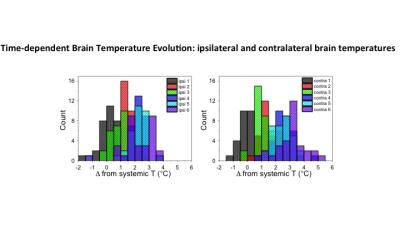
Representative NHP brain temperature histogram corresponding to figure 1, and obtained by non-invasive MR thermometry following stroke induction. X-axis indicates brain temperature offset relative to contemporaneous systemic (rectal) temperatures in the ipsilateral (ipsi) and contralateral (contra) hemispheres. Color assignments correspond to six normalized time points following stroke induction, demonstrating progressively increasing brain temperatures, outpacing systemic febrile response following stroke induction.

Brain-systemic temperature offset presented in pre-stroke physiologic and post-stroke (seven hours post-induction) thermographs. Brain-systemic temperature differences indicate increasing brain temperatures following infarction, exceeding systemic temperature changes. Diffusion weighted image (DWI, far right) obtained at conclusion of stroke induction day demonstrating right middle cerebral artery territory infarction.