4717
Determinants of the Presence and Intensity of Hyperintense Vessels on Fluid-Attenuated Inversion Recovery Imaging in Intracranial Atherosclerotic Stenosis or Occlusion1Radiology, Chinese PLA General Hospital, Beijing, People's Republic of China, 2Beijing Tiantan Hospital, Capital Medical University, Beijing, People's Republic of China, 3Radiology, Chinese PLA General Hospital, People's Republic of China, 4GE healthcare China, People's Republic of China
Synopsis
The correlation of fluid-attenuated inversion recovery imaging (FLAIR) vascular hyperintensity (FVH) and collateral are still in discrepancy in intracranial atherosclerotic stenosis or occlusion. In the present study, we used territorial arterial spin labeling (ASL) and two post labeling delay (1.5s and 2.5s) ASL to study the leptomeningeal collateral and hemodynamic status in patients with different intensity of FVH, and concluded that FVH may be related to poor collateralization and hemodynamic impairments.
Introduction
Fluid-Attenuated Inversion Recovery Imaging (FLAIR) vascular hyperintensity (FVH) were considered resulting from hemodynamic changes other than thrombosis and as a marker of slow flow leading absence of flow void in patients with intracranial atherosclerotic stenosis or occlusion. The correlation of FVH and collateral are still in discrepancy in this disease1. Three dimensional pseudo-continuous arterial spin labeling (3D pCASL) technique including territorial ASL and multiple post labeling delay (PLD) ASL are promising approaches to study collateral circulation2. The present study was to investigate the determinants of presence and intensity of FVH sign and thus its indication for collateral circulation by ASL perfusion imaging in patients with unilateral middle cerebral artery atherosclerotic stenosis or occlusion.Methods
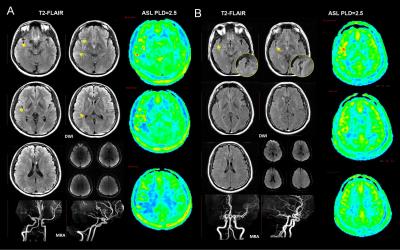
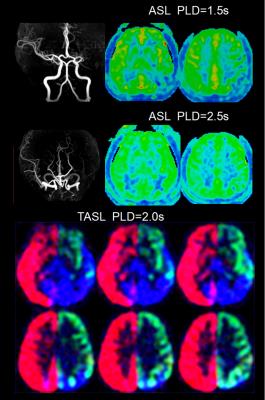
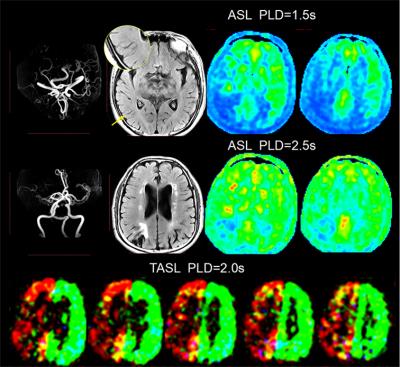
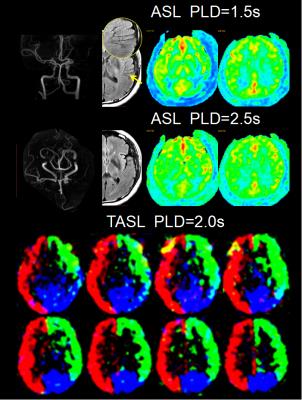
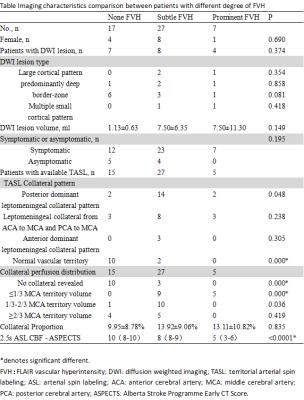
From October 2015 to June 2016, we prospectively enrolled consecutive patients with unilateral middle cerebral artery(MCA) atherosclerotic severe stenosis(70%-99%) or occlusion confirmed by MR angiography (MRA). Demographics of patients including risk factors, time from symptom onset to MRI scanning were recorded. All patients were performed territorial ASL (PLD=2.0s), two PLD (1.5s and 2.5s) 3D pCASL, T2FLAIR (slice thickness = 5mm), MRA and diffusion weighted imaging (DWI) on a 3.0T scanner(GE MR 750, USA). Cerebral blood flow (CBF) maps of TASL and 3D pCASL were post-processed on AW4.5 Workstation (GE Healthcare, China). FVH was defined as a linear or serpentine hyperintense appearance on FLAIR distal to the stenosis or occlusive lesion in line with a typical arterial course. The Intensity of FVH was graded as none, subtle and prominent. Continuous FVH presenting on more than 4 slices or greater than 1/3 downstream territory was considered as prominent and FVH presenting on less than 4 slices and 1/3 downstream territory considered as subtle. Figure 1 showed two examples with prominent (A) and subtle (B) FVH. DWI lesion volume were measured and lesion pattern were assessed based on previous study3. Collateral perfusion proportion were calculated according to a previous study using two PLD 3D pCASL approach4, and meanwhile 2.5s CBF Alberta Stroke Programme Early CT Score (ASPECTS) were quantified in accordance to the original study5. Degree of leptomeningeal collateral distribution was assessed as ≤ 1/3, 1/3-2/3, and ≥2/3 MCA territory volume by 2.5s ASL-CBF map subtracting 1.5s ASL-CBF map based on our previous study6. Leptomeningeal collateral pattern were then assessed by TASL as shown in Figure 2-4. DWI lesion, collateral perfusion proportion, leptomeningeal collateral pattern and demographics were compared between groups with different intensity of FVH using ANOVA or non-parametric and X2 test.Results
We totally enrolled 51 patients(57.15±10.73 years, 13 females) with 7 patients with prominent FVH, 27 patients with subtle FVH and 17 patients without FVH. Clinical materials including gender, risk factors and time from symptom onset to MRI scanning were not significant different between the 3 groups (not shown in Figure 5). In table of Figure 5, it is seen that patients with normal vascular territory distribution and no collateral compensation were most frequently observed in patients without FVH, patients with collateral perfusion less than 1/3 MCA territory were most frequently observed in patients with prominent FVH. Additionally, 2.5s CBF-ASPECTS were significantly lower in patients with prominent FVH and patients with subtle FVH were significantly lower than patients without FVH.Discussion and Conclusion
In unilateral middle cerebral artery atherosclerotic stenosis or occlusion, collateral recruitment and degree of collateral perfusion distribution may be associated with the presence and intensity of FVH. FVH are more prevalent in patients with poor collateralization. Patients with more prominent FVH may have experienced more severe hypoperfusion, which indicates that the intensity of FVH may be an useful marker of cerebral hymodynamic impairments.Acknowledgements
No acknowledgement found.References
1.Azizyan A, Sanossian N, Mogensen MA, Liebeskind DS. Fluid-Attenuated inversion recovery vascular hyperintensities: An important imaging marker for cerebrovascular disease. Am J Neuroradiol. 2011;32:1771-1775.
2.Hartkamp NS, van Osch MJP, Kappelle J, Bokkers RPH. Arterial spin labeling magnetic resonance perfusion imaging in cerebral ischemia. Curr Opin Neurol. 2014;27:42-53.
3.Bang OY, Saver JL, Alger JR, Starkman S, Ovbiagele B, Liebeskind DS, et al. Determinants of the distribution and severity of hypoperfusion in patients with ischemic stroke. Neurology. 2008;71:1804-1811.
4.Lyu J, Ma N, Liebeskind DS, Wang DJJ, Ma L, Xu Y, Wang T, Miao Z, Lou X. Arterial spin labeling magnetic resonance imaging estimation of antegrade and collateral flow in unilateral middle cerebral artery stenosis. Stroke. 2016;47:428-433.
5.Aviv RI, Mandelcorn J, Chakraborty S, Gladstone D, Malham S, Tomlinson G, et al. Alberta stroke program early ct scoring of ct perfusion in early stroke visualization and assessment. AJNR Am J Neuroradiol. 2007;28:1975-1980.
6.Lyu J, Ma N, Miao Z, Ma L,Lou X. Validity of Three Dimensional Pseudo-Continuous Arterial Spin Labeling in Leptomeningeal Collaterals Assessement for Patients with Unilateral Middle Cerebral Artery Stenosis. ISMRM 23rd Annual Meeting & Exhibition, 2015, Toronto, Canada.
Figures